PAM Review: Energy Science & Technology, Vol. 7, 2020
ISSN 2205-5231 | Published by UTS ePRESS | https://epress.lib.uts.edu.au/student-journals/index.php/PAMR/index
A parametric analysis on PEFCs for high-temperature applications
Filip Bojko1,*, Giorge Gemisis2, James Mitchell3, Christopher Parker4
University of Technology Sydney, Faculty of Science, PO Box 123, Ultimo NSW 2007, Australia
1Filip.Bojko@student.uts.edu.au
2Giorge.Gemisis@student.uts.edu.au
3James.Mitchell@student.uts.edu.au
4Christopher.J.Parker@student.uts.edu.au
* Author to whom correspondence should be addressed.
DOI: https://doi.org/10.5130/pamr.v7i0.1594
Citation: Bojko, F., Gemisis, G., Mitchell, J., Parker, C. 2020. A parametric analysis on PEFCs for high-temperature applications. PAM Review: Energy Science & Technology, 7, Article ID 1594. https://doi.org/10.5130/pamr.v7i0.1594
© 2020 by the author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License (https://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Abstract:
Polymer Electrolyte Fuel Cells (PEFCs) are an increasingly significant facet of modern renewable energy and transportation, providing an electrochemical method of energy generation with high power density, thermal properties, and efficiency. PEFCs tend to increase in efficiency as temperature increases but detrimental effects begin to occur, including membrane degradation and dehydration. These effects are unfavourable in the design of optimised fuel cells as they can result in reduced efficiency and lifetime. Current PEFCs are in a state where they are commercially viable but have a very limited temperature operation region (<80°C). This meta-study analysis presents research around expanding the operational temperatures of PEFCs through a parametric analysis of active cell area, phosphonic acid content, and organic/inorganic fillers. This analysis finds an increase in proton conductivity for PEFCs at higher temperature by using phosphonic acid functionalised membranes with maximised degree of phosphonation (up to 1.5 DP). It was also found that using ionic liquid functionalised carbon materials as fillers was an effective strategy to enhance the proton conductivity of PEFCs in a higher temperature environment while also providing increased thermal stability of the membrane. Additionally, higher thermal efficiency and power density may be achieved by increasing temperature and humidity to maximise proton conductivity towards theoretical maxima dictated by the active cell area, which was found to peak at 36 cm2.
Keywords:
High-Temperature; Fuel Cell; PEFC; Proton Conductivity, Phosphonic, Electrolyte
Nomenclature
1. Introduction
High-Temperature Polymer Electrolyte Fuel Cells (HT-PEFCs) are a major subset of current fuel cell technology, representing an active field of research and development over the past two decades. [1-3,5,7] This is because they provide an original form of electromechanical energy generation, with higher efficiency, power density, and less demanding fuel purity requirements than low temperature variants [1,2]. Typically, a low temperature PEFC will operate below 80°C, whereas high temperature variants operate between 100°CC – 200°C [2]. Although this provides advantageous properties in a stable system, the elevated operating temperature impacts the thermal, chemical, and mechanical stability of polymers, and provides a significant challenge in obtaining stable proton conductivity under ambient conditions [2,3]. This paper provides a meta-study into the current research surrounding HT-PEFC development, and provides evidence indicating that the manipulation of phosphonic acid content, active cell area, and the addition of filler materials can achieve higher efficiency in PEFCs with similar polymer electrolyte membrane (PEM) composition.
1.1. Fuel Cell Overview
A major constituent of a PEFC is the proton conducting PEM, a semipermeable membrane commonly composed of ionomers, that simultaneously acts as a proton conductor and electrical insulator. This PEM operates between an anodic and cathodic layer, which are bridged through output lines to form a complete electrode. The PEM is then encased alongside an electrolyte, catalyst, and gas diffusion layer to form an assembled stack known as the membrane electrode assembly (Fig. 1).
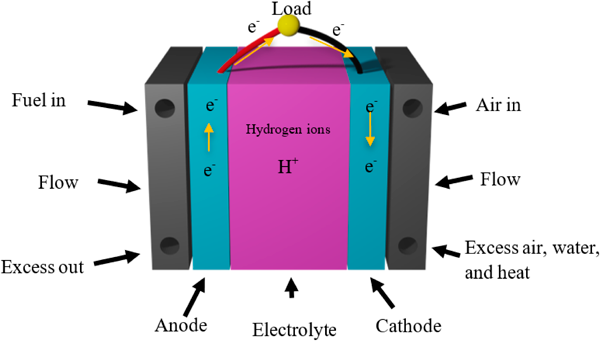
Figure 1 PEFCs operate with the injection of a fuel source (commonly diatomic hydrogen) and an oxidant into the MEA system through a set of bipolar plates containing gas supply channels. A gas diffusion layer is formed about the anode and cathode to manage by- product water and prevent saturation of the chamber. The oxidant is fed towards the cathode, while the fuel is fed towards the anode [4].
In PEFC operation, a catalyst is used to separate electrons and protons from a fuel source; in the case of H2, this refers to the separation of hydrogen ions and electrons. The hydrogen ions t1.hen travel through the PEM. This leaves the freed electrons to follow the external path provided between the anode and cathode to power external loads. Once the protons, electrons and oxidants meet at the cathode they combine, generating heat and creating water as a by-product [3,5,6]. The two chemical half equations that describe this process are as follows:

The effect of creating these by-products is known as the humidification of the MEA assembly, where the management of the current percentage water volume is a major consideration in current literature [2]. It is vital to balance the humidity of the fuel cell to maintain high conductivity and ensure membrane hydration, without reaching a level where water saturation occurs and disrupts gas channels.
1.2. Electrochemistry
The electrical properties of a PEFC are determined by several contributing factors, these factors include things such as catalyst composition, active cell areas, oxygen reductions reaction kinetics, phosphoric acid content and the presence of organic/inorganic fillers. Catalysts are essential in the chemical reaction to facilitate the separation of protons and electrons. The magnitude of proton conductivity is governed by the Arrhenius equation:
Where the proton conductivity σ is a function of the initial proton conductivity of the activation energy, universal gas constant, and absolute temperature. As there is a direct correlation between proton conductivity and efficiency in the form of current density, there is an observable relationship between the operational temperature of the PEFC and the output capacity [2,8]. This also indicates that a lower activation energy is required in high temperature operation. However, catalysts will also react with impurities in the fuel, causing Carbon Monoxide poisoning in the MEA and impacting the efficiency of the fuel cell [7]. This effect is potentially magnified at higher operating temperatures.
Proton conductivity is also determined by the effective cell area and the resistance of the membrane. This is determined by the following equation:
Where L is the thickness, R is the resistance, and A is the surface area between membrane and electrode [9].
1.3. Thermodynamics
The heat produced from over-voltage in the fuel cell must be removed otherwise overheating of the fuel cell can occur and have a detrimental effect on the efficiency. Over-voltage occurs when excess voltage is supplied, and the excess electrical energy is consequently converted to heat energy. To counteract this, a reversible cell potential is applied to the fuel cell. Eq. 5 relates the reversible cell potential to changes in the Gibbs function dependent on the fuel cell operating temperature and pressure.
This indicates that cell potential varies with temperature based on the change in entropy for the fuel cell electrochemical rection. In situations where ΔS > 0, reversible cell potential will increase with temperature resulting in greater power density.
However, increasing the temperature indefinitely will begin to have detrimental effect on the fuel cell efficiency, as material decay will prohibit the stability of the electrochemical reaction and affect the lifetime of the fuel cell [10,11]. The focus was to find a set of variables that better allow the fuel cell to work at higher temperatures and not suffer the decay in efficiency due to the materials. The proton conductivity affects the way the protons move through the membrane if the proton conductivity is higher, then the fuel cell will have less ohmic drop and this higher efficiency. These follow the equations outlined below:

The ohmic drop is proportional to the current density and the resistance area of the membrane. The resistance area is dependent on the area of the membrane and the proton conductivity. This is combined in the form of the voltage efficiency where the efficiency relies on the reversible voltage, the ohmic drop and the thermodynamic voltage.
This indicates that higher efficiency in PEFCs is achievable by implementing larger proton conductivity and higher operating temperatures [12].
Proton conductivity is one of the most important factors in characterising polymer electrolyte membranes for their use in fuel cell applications as it directly relates to the efficiency of the fuel cell [13]. The mechanism by which the protons diffuse through the membrane is known as the Grotthuss mechanism (Fig.2)
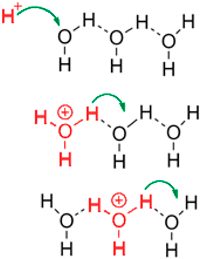
Figure 2 A schema of the Grotthuss mechanism, where an excess proton diffuses through the hydrogen bond network of water molecules. This happens through the formation and cleavage of covalent bonds with adjacent hydrogen bonded molecules of water and effectively causes the proton to jump between molecules. This works not only in water but in other hydrogen bonded liquids in general.
There are multiple factors that affect proton conductivity in the membranes such as ion exchange capacity (IEC), polymer morphology, relative humidity of the membrane (RH), and temperature [15]. From these factors listed IEC is an essential factor in determining the proton conductivity of a membrane, it is a measure of the molar equivalents of ion conductors available per unit mass of the dry polymer membrane [16]. In general, a higher IEC is required to achieve high proton conductivity (e.g., IEC > 2.0 meq/g) is generally required [15].
Ideally, a membrane material that can operate at higher temperatures in a low humidity environment and has a moderately high proton conductivity (high IEC) is therefore widely considered to be the most crucial element to further progress in PEMFC technology, a number of potential solutions have been proposed to achieve a PEM that has these properties [17]. For the commercial perfluorosulfonic acid membrane (e.g., a Nafion membrane) it is well known that the proton conductivity is mainly a function of temperature and relative humidity [18]. At higher temperatures, the proton conductivity decreases, this is due to the dependence of the perfluorosulfonic acid membrane on water to facilitate the proton conduction through the Grotthuss mechanism. Consequently, they are heavily dependent on a high humidity/water content environment and at these temperatures the membranes experience dehydration, resulting in low proton conductivities. As such their operating range is limited to <80°C [19]. Due to this, commercial Nafion series membranes would not be the most ideal for use in high temperature applications as it has a high dependence on humidity and as such a better alternative is preferable.
Several methods of improving on existing membrane technology to develop one with more desirable properties for high temperature applications have been proposed, one such method of achieving this is the construction of hybrid acid-base polymer membranes, these membranes exhibit higher proton conductivity at higher temperatures (100°C – 200°C) [20]. This works due to more proton donor/acceptor molecules being present to facilitate the diffusion of the proton reducing the membranes reliance on the humidity/water content that was enabling the Grotthuss mechanism to begin with.
Schuster et al. [17] concluded that the phosphonic acid group was the most advantageous acid group to use in the construction of hybrid acid-base membranes due to its amphoteric nature and molecular structure causing it to act as an immobilised proton solvent in the membrane. This results in high proton mobility under low humidity conditions at intermediate temperatures (120°C – 160°C) as the need for water to encourage proton conduction is replaced by the presence of the phosphonic acid groups. The actual construction of these membranes is most reliably done through chemical grafting of phosphonic acid groups to a polymer of choice, several different types of polymers such as poly(styrene), poly(phenylene oxide), poly(imide), and poly(sulfone) have been used to create HT-PEM, some of the results of these studies have been summarised in Table 1.
Another common method of overcoming the loss of proton conductivity due to dehydration is by adding proton conductive fillers to the polymer membrane in order to increase the electric properties independently from the water content. Fillers in general are solid particles that are either fine or fibrous in nature which are added to the fuel cells membrane to improve specific properties like heat resistance, chemical and mechanical properties, moisture absorbance or electrical characteristics. Usually, fillers are classified into two different categories: (1) organic and (2) inorganic fillers. In this study, we have focused on those with effects on the electrical properties such as the proton conductivity, or the water retainability. Furthermore, both organic and inorganic fillers are compared.
2. Methods
The goal of this meta study was to collate the findings from other scientific literature regarding the effect of operational parameters on the thermodynamic properties of commercial high-temperature polymer electrolyte fuel cells. The literature for this meta-analysis was obtained from academic databases, namely SCOPUS, Springer, and Science Direct. Furthermore, to ensure the continued relevance of literature and information used within the study, search parameters were limited to a timeframe of 10 years in order to prioritise the most recent advances in the research area. However, papers outside this timeframe were still considered if the research covered was still the most recent contribution in that area. Keywords were used to further narrow the scope of research for review. These keywords include: High-Temperature, Stability, Degradation, Proton Conductivity, and Thermodynamic.
The operational definition of HT-PEFC was taken as the definition provided by the Department of Energy Conversion and Storage; A fuel cell composed of a PEM and cells operating between the temperature range of 100°C – 200°C [2]. This does not include the molten carbonate and solid oxide subcategory of fuel cells which operate at much higher temperatures. The data indicated a research emphasis towards parameters including membrane phosphonic acid content, active cell area, oxygen reduction reaction kinetics, and organic/inorganic fillers. Literature on the effect of these parameters and the effect of operating temperature on them was compiled and analysed in Excel to identify consistent trends and potential avenues for the continued improvement of thermodynamically stable HT-PEFCs. Where datasets were not made available, data was extracted from figures using the WebPlotDigitizer application and collected into Excel for plotting.
3. Results and Discussion
3.1. Humidity and Proton Conductivity
Li et al. states that the correlation between internal humidity and proton conductivity can be attributed to the dual role of water as a free proton carrier in high temperature MEA assemblies.
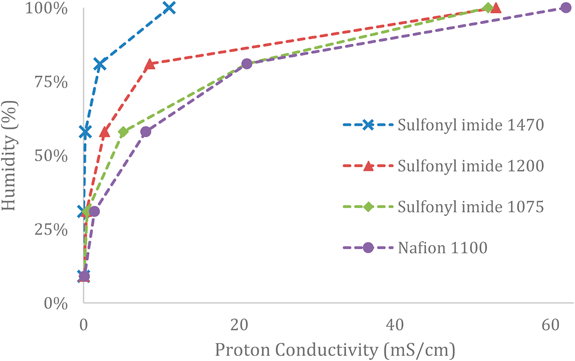
Figure 3 Proton Conductivity as a function of Humidity for HT-PEFC (% Water content of the MEA catalyst) [21, 22]. This effect can be seen consistently across various membrane compositions is indicative of the known relationship between proton conductivity and the humidification effect.
Figure 3 exhibits this relationship across multiple sulfonic polymer compositions and supports the idea that PEFCs operating at temperatures above 100°C benefit from the increase of free carriers in the MEA and are not subject to gas channel disruption. This effect results in a value for proton conductivity that approaches the theoretical maximum dictated by the geometric properties of the cell as governed by Eq. 4.
3.2. Effect of Active Cell Area on Efficiency
Active cell area is the part of the fuel cell that is next to the flow field from this means the cell area relies heavily on how efficient the flow field is in allowing the diffusion of fuels in it. The measurements above all use the same type of flow field using a serpentine flow field, as seen in Figure 4b, as it’s seen to be an efficient way of moving reactants in the MEA and into the gas diffusion layer [23]. Furthermore, the voltage used in each measurement was 0.6 V to keep a constant in all the results.
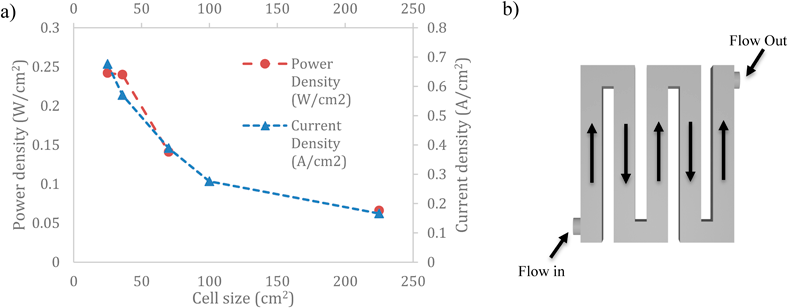
Figure 4 (a) Power Density and Current Density as a function of active cell area [24,31,32]. (b) A model on serpentine flow field geometry
The current density and power density have a direct correlation between them and the cell size, as seen in Figure 4a. This implies an upper limit to cell size before the cell begins to become inefficient [24]. The active cell area influences the overall efficiency of the PEFC as seen in Eq 8. which indicates that a smaller cell area has a knock-on effect on the Ohmic Drop Eq. 6. that then has a positive effect on the overall thermodynamic voltage. In Figure 4a this relationship mostly holds true until 36 cm2 where it reaches a point of diminishing returns, where the power density begins to plateau.
This plateau may be attributed to the flow field beginning to shrink due to the cell size getting smaller. This causes the water to flow under increasing resistance through the flow field and sticking to the sides of the flow field impeding the travel. The fuels also begin to get stuck in the corners or stuck in slight imperfections of the flow field [23,24,31,32]. Another factor is pressure drop, if the pressure is not kept at a constant flow, the flow will become turbulent and not laminar and restrict the free movement of fuel, impacting the efficiency of the geometry chosen [23]. Overall, this shows for a new type of cell, an active area of less than 36 cm2 will have little to no effect on the power density of the cell [24].
3.3. Phosphonic Acid Composition
As discussed earlier, a higher IEC is required in general to achieve high proton conductivity, as such there is a direct correspondence between IEC and proton conductivity of the membrane. A more tangible relationship for characterising a polymer for its use in a specific application is the effect of the degree of membrane phosphonation (DP) on the IEC of the polymer. Figure 5 shows this relationship. (The DP of a polymer is a measure of the number of repeating functional units of phosphonic acid per repeating unit of the polymer).
Table 1. Shows several different phosphonic acid functionalised membranes from different studies and compares the maximum proton conductivity achieved by each and at what temperature it was reached, from this table it is clear that some polymers performance as PEMs when phosphonated is much more suitable for the high temperature applications we are studying, such as poly(sulfone) and poly(styrene-ethylene-butylene-styrene) (Poly(S-E-B-S)) which both achieved moderately high conductivity in an appropriate temperature range according to their respective studies [27,9].
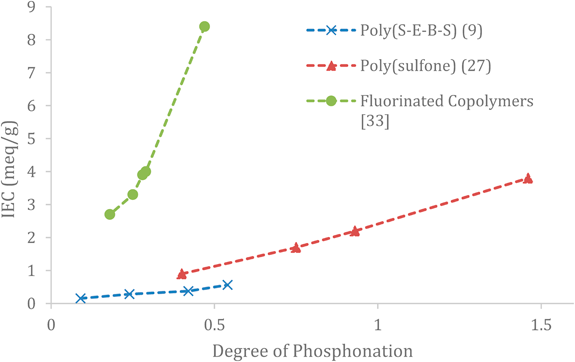
Figure 5 IEC of various polymers as a function of the number of repeating functional units of phosphonic acid per repeating unit of the polymer (Degree of Phosphonation) [9, 27, 33].
In the study undertaken by Parvole and Jannasch [26], Poly(sulfones) had side chains of poly(vinyl phosphonic acid) (PVPA) grafted to them. Poly(sulfone) with up to 57wt% PVPA was measured, they found this to have the highest IEC at 5.3meq/g. This study found that in general the higher weight percentage of the polymer that was PVPA the higher the IEC of the membrane and hence the higher the proton conductivity of the membrane, this result provides positive affirmation to the trend seen in the data of Fig 5.
Elumalai et al. [9] explored multiple different degrees of phosphonated Poly(S-E-B-S), these were constructed and tested at 140°C. Their study found that 54% phosphonated P(S-E-B-S) achieved the highest proton conductivity at this temperature of 5.81mS/cm, they concluded that the IEC of the membranes increases with increasing degree of phosphonation of the membrane, and hence the proton conductivity increases. Studies by Abu-Thabit et al., Sun et al., and Tayouo et al. [27,28,33] also all reported that the synthesised membranes with the highest phosphonic acid content reached the highest proton conductivity, with Abu-Thabit et al. [27] testing poly(sulfone) membranes with up to 1.5 DP.
From the gathered data it may be concluded that the degree of phosphonation should be maximised to achieve the highest proton conductivity. This is expected, since more proton donor/acceptor molecules being present would evidently mean higher IEC and hence higher proton conductivity - this would reduce the dependence of the membranes on the hydration/humidification being the enabler to the Grotthuss mechanism. The ability of these phosphonate functionalised polymers to still maintain moderate proton conductivites at lower degrees of hydration makes them particularly attractive for electrochemical applications [26] as it provides a viable method to help subdue issue of membrane dehydration at higher operating temperatures of the PEM.
3.4. Fillers
Ye et al. [29] studied ionic liquid functionalised carbon materials (IL-FCM), 0D carbon black (CB), 1D multi-walled carbon nanotubes (MWCNT) and 2D reduced graphene oxide sheets (RGO), incorporated into a composite polymer electrolyte, sulfonated polyimide (SPI). They observed a higher proton conductivity for all three materials, especially at higher temperatures. Figure 6 shows the proton conductivity for each carbon material and the pristine membrane measured for temperatures up to 160°C. The highest conductivity (7.8 S/cm) measured was for the graphene sheets (FG) at a temperature of 160°C, which may be due to the higher surface area of the (2D) graphene sheets, enhancing the filler/IL interaction. In general, we can see an increase in proton conductivity at increasing temperatures, which may indicate that the effect is independent of filler composition.
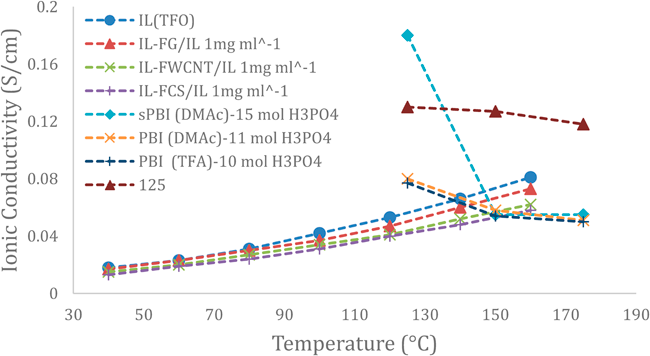
Figure 6 Ionic conductivity of the membrane incorporating different IL-fillers. (FG: functionalized graphene; FWCNT: functionalized walled carbon nanotubes; FCS: functionalized carbon black, [27,28].
The conductivity has been measured through-plane and in-plane. The in-plane measurement revealed an increase in proton conductivity of the IL-FG/IL for increasing carbon loadings whereas the conductivity was decreasing for IL-FWCNT/IL and IL-FCS/IL with increasing carbon loadings. The through-plane measurement on the other hand side shows a trend, where the improvement is highest for a medium loading of 0.4wt%. The group has measured the improvements only for three different values of carbon loading, therefore there might be a greater increase in conductivity for another value between 0.2wt%-0.8wt%. Also, in this case IL-FG/IL showed the highest conductivity.
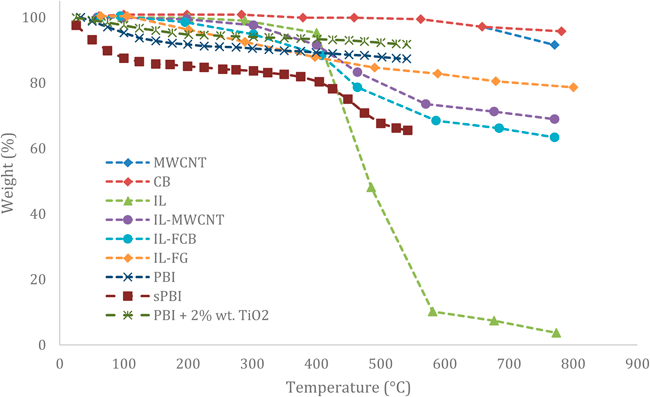
Figure 7 TGA curves of IL (TFO), CMs, IL-FCMs, and non-doped PBI-films (including a composite TiO2). There is a characteristic decline across datasets in temperatures greater than 200°C [29, 30].
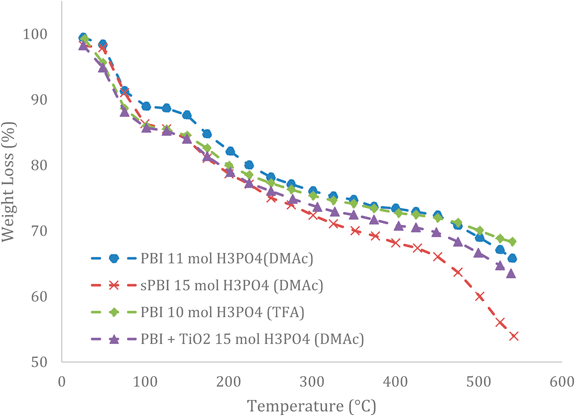
Figure 8 TGA curves for doped PBI-films (including a composite TiO2) [30].
Lobato et al. [30] Published an even higher improvement of the proton conductivity for HT-PEMFC by using TiO2 in a poly(benzimidazole) (PBI) membrane with H3PO4. The highest water retention capacity as well as the highest conductivity was measured for a temperature of 125°C at a level of 11.7 × 10 S/cm. The research showed that the proton conductivity for doped TiO2-PBI membranes is independent of temperature variation (Values stayed maintain around 10 × 10-2 S cm-1 for temperatures up to 160°C).
Furthermore, this filler showed a higher water retention capacity and a higher power density, even at higher temperatures (800 mW/cm2), which was without any precedent in other literatures by the date of publishing. They interpreted this behaviour because of the highly acidic sites absorbing water on the surface. Lobato et al. mentioned further issues (mechanical properties and FC performances under different conditions) for this filler type. However, comparing the fillers of both studies reveals a higher thermal stability for the nanostructured carbon functionalised IL than for the TiO2. Fig. 7 and 8 shows the weight loss of each membrane type by a thermogravimetric analysis. The IL-FCM/IL filler have a higher thermal stability than the TiO2, but both are suitable for high temperature applications. Additionally, Fig. 7 shows the TGA curve of the non-doped PBI-films.
Comparing the fillers studied by Ye et al. and Lobato et al. [29,30], we can say that the proton conductivity for both filler types is promising for future technologies. However, further properties like the mechanical stability as well as the performance at different temperatures and under different conditions seems to be better in the case of IL-FCMs, even though the TiO2 yields a higher proton conductivity. In contrast to the TiO2, IL-FCMs have not been studied by using doped membranes as well. The study of Lobato et al [30] indicates, that examining the ion conductivity and the performance of SPI- IL-FCM membranes by H3PO4 doping is promising as it has not only improved the thermal stability of the TiO2 membrane but is also known for good water retention properties H3PO4 might increase the conductivity of SPI-IL-FCM membranes additionally as the IL-FCMs increase the conductivity of the membrane independently from the water content in the cell and H3PO4 on the other hand yields a good water retention capacity, which leads to an increase in conductivity respectively.
Conclusions
The meta-analysis conducted in this paper has observed promising avenues to explore in the ongoing realm of HT-PEFC research and development. A parametric analysis of research on the areas of the humidification effect, cell area, phosphonic acid PEMs, and filler composition has indicated that there is the possibility to combine aspects to increase efficiency. The previous discussion has shown that the known correlation between humidification and proton conductivity in PEFCs operating above 100°C leads to a direct relationship between operating temperature and power density in electrochemical reactions with a positive entropy. This property is seen to approach theoretical maximums dictated by the cell area. In this way, higher thermal efficiency and power density may be achieved by balancing humidity in PEFCs with a cell area small enough to achieve a positive cascading effect on thermodynamic voltage, while large enough to avoid issues with cavitation and turbulent flow. The peak value for this was found to be approximately 36cm2, with further reduction in cell area resulting in no measurable effect on power density.
Further consideration was given to phosphonic acid functionalised PEM compositions, from the analysis performed it was concluded that the degree of membrane phosphonation should be maximised to achieve the highest proton conductivity of the PEM. The meta-study found that membrane phosphonation may be an avenue in which the membranes reliance on the humidification can be reduced, and hence improve their suitability for higher temperature applications.
This meta-analysis also examined the effect of membrane augmentation by organic and inorganic fillers, it was found that these fillers can improve multiple critical aspects of the PEFC, namely proton conductivity and mechanical stability at various temperatures. The us of ionic liquid functionalised carbon materials as fillers was found to be an effective strategy to enhance the proton conductivity of PEFCs in a higher temperature environment. These IL-FCM membranes were measured to have lower proton conductivities compared to the doped TiO2 PBI membranes. However, they exhibited higher mechanical stability at higher temperatures and as such IL-FCM membranes may be a promising path forward in developing a PEM that is much more optimised for high temperature applications.
The analysis in this study suggests that future research should consider examining the practicality of IL-FCM fillers incorporated with phosphonic acid doped membranes in a PEFC using the peak cell area of 36cm2, this may provide a good basis for a HT-PEFC to which further optimisations can be developed.
Acknowledgments
The authors would like to acknowledge the guidance provided by Dr. Jurgen Schulte, Blake Regan, Brendan Boyd-Weetman, and the staff of the UTS Library.
References and Notes
[1] Engl T, Gubler L, Schmidt TJ. Think Different! Carbon Corrosion Mitigation Strategy in High Temperature PEFC: A Rapid Aging Study. J Electrochem Soc 2015;162(3):F291-F297. https://doi.org/10.1149/2.0681503jes
[2] Li Q, Aili D, Hjuler HA, Jensen JO. High Temperature Polymer Electrolyte Membrane Fuel Cells: Approaches, Status, and Perspectives. 1st ed. Cham: Springer International Publishing; 2016. https://doi.org/10.1007/978-3-319-17082-4
[3] Pasupathi S. Recent advances in high-temperature PEM fuel cells. Amsterdam: Elsevier; 2016.
[4] Tong S, Qian D, Huo C. Hydrogen-air PEM fuel cell : integration, modeling, and control. Berlin ;: Walter de Gruyter GmbH; 2018. https://doi.org/10.1515/9783110602159
[5] Eikerling M, Kulikovsky A. Polymer Electrolyte Fuel Cells: Physical Principles of Materials and Operation. : CRC Press; 2014. https://doi.org/10.1201/b17429
[6] Lee HS, Kim HJ, Kim SG, Ahn SH. Evaluation of graphite composite bipolar plate for PEM (proton exchange membrane) fuel cell: Electrical, mechanical, and molding properties. Journal of Materials Processing Tech 2007;187(1-3):425-428. https://doi.org/10.1016/j.jmatprotec.2006.11.213
[7] Aricò AS, Stassi A, Modica E, Ornelas R, Gatto I, Passalacqua E, et al. Performance and degradation of high temperature polymer electrolyte fuel cell catalysts. J Power Sources 2008;178(2):525-536. https://doi.org/10.1016/j.jpowsour.2007.10.005
[8] Fang J. Polyimide Proton Exchange Membranes. 2018:323-383. https://doi.org/10.1016/b978-0-12-812640-0.00007-x
[9] Elumalai V, Annapooranan R, Ganapathikrishnan M, Sangeetha D. A synthesis study of phosphonated PSEBS for high temperature proton exchange membrane fuel cells. J Appl Polym Sci 2018;135(10). https://doi.org/10.1002/app.45954
[10] Schalenbach M, Carmo M, Fritz DL, Mergel J, Stolten D. Pressurized PEM water electrolysis: Efficiency and gas crossover. International Journal of Hydrogen Energy 2013;38(35):14921-14933. https://doi.org/10.1016/j.ijhydene.2013.09.013
[11] Wei G, Xu L, Huang C, Wang Y. SPE water electrolysis with SPEEK/PES blend membrane. Int J Hydrogen Energy 2010;35(15):7778-7783. https://doi.org/10.1016/j.ijhydene.2010.05.041
[12] Schalenbach M, Carmo M, Fritz DL, Mergel J, Stolten D. Pressurized PEM water electrolysis: Efficiency and gas crossover. Int J Hydrogen Energy 2013;38(35):14921-14933. https://doi.org/10.1016/j.ijhydene.2013.09.013
[13] Lee CH, Park HB, Lee YM, Lee RD. Importance of Proton Conductivity Measurement in Polymer Electrolyte Membrane for Fuel Cell Application. Ind Eng Chem Res 2005;44(20):7617-7626. https://doi.org/10.1021/ie0501172
[14] Ludueña GA, Kühne TD, Sebastiani D. Mixed Grotthuss and Vehicle Transport Mechanism in Proton Conducting Polymers from Ab initio Molecular Dynamics Simulations. Chem Mater 2011;23(6):1424-1429. https://doi.org/10.1021/cm102674u
[15] Liaw D, Wang K, Huang Y, Lee K, Lai J, Ha C. Advanced polyimide materials: Syntheses, physical properties and applications. Progress in Polymer Science 2012;37(7):907-974. https://doi.org/10.1016/j.progpolymsci.2012.02.005
[16] Elumalai V, Annapooranan R, Ganapathikrishnan M, Sangeetha D. A synthesis study of phosphonated PSEBS for high temperature proton exchange membrane fuel cells. J Appl Polym Sci 2018;135(10):45954. https://doi.org/10.1002/app.45954
[17] Schuster M, Rager T, Noda A, Kreuer KD, Maier J. About the Choice of the Protogenic Group in PEM Separator Materials for Intermediate Temperature, Low Humidity Operation: A Critical Comparison of Sulfonic Acid, Phosphonic Acid and Imidazole Functionalized Model Compounds. Fuel Cells 2005;5(3):355-365. https://doi.org/10.1002/fuce.200400059
[18] Zhang J. PEM fuel cell testing and diagnosis. Amsterdam: Elsevier; 2013.
[19] Heo P, Shen Y, Kojima K, Pak C, Choi KH, Hibino T. Fe0.4Ta0.5P2O7-based composite membrane for high-temperature, low-humidity proton exchange membrane fuel cells. Electrochim Acta 2014;128:287-291. https://doi.org/10.1016/j.electacta.2013.08.107
[20] Yamada M, Honma I. Anhydrous protonic conductivity of a self-assembled acid-base composite material. J Phys Chem B 2004;108(18):5522-5526. https://doi.org/10.1021/jp030767o
[21] Savett SC, Atkins JR, Sides CR, Harris JL, Thomas BH, Creager SE, et al. A Comparison of Bis[(perfluoroalkyl)sulfonyl]imide Ionomers and Perfluorosulfonic Acid Ionomers for Applications in PEM Fuel-Cell Technology. J Electrochem Soc 2002;149(12):A1527. https://doi.org/10.1149/1.1516218
[22] Savadogo O. Emerging membranes for electrochemical systems: Part II. High temperature composite membranes for polymer electrolyte fuel cell (PEFC) applications. J Power Sources 2004;127(1):135-161. https://doi.org/10.1016/j.jpowsour.2003.09.043
[23] Su A, Weng F, Hsu C, Chen Y. Studies on flooding in PEM fuel cell cathode channels. International Journal of Hydrogen Energy 2006;31(8):1031-1039. https://doi.org/10.1016/j.ijhydene.2005.12.019
[24] Karthikeyan M, Muthukumar M, Karthikeyan P, Mathan C. Optimization of active area of proton exchange membrane fuel cell with better water management. Journal of Ceramic Processing Research 2019;20(5):490-498. https://doi.org/10.36410/jcpr.2019.20.5.490
[25] Meng YZ, Tjong SC, Hay AS, Wang SJ. Synthesis and proton conductivities of phosphonic acid containing poly‐(arylene ether)s. Journal of Polymer Science Part A: Polymer Chemistry 2001;39(19):3218-3226. https://doi.org/10.1002/pola.1304
[26] Parvole J, Jannasch P. Polysulfones grafted with poly(vinylphosphonic acid) for highly proton conducting fuel cell membranes in the hydrated and nominally dry state. Macromolecules 2008;41(11):3893-3903. https://doi.org/10.1021/ma800042m
[27] Abu-Thabit NY, Ali SA, Javaid Zaidi SM. New highly phosphonated polysulfone membranes for PEM fuel cells. Journal of Membrane Science 2010;360(1):26-33. https://doi.org/10.1016/j.memsci.2010.04.041
[28] Sun J, Jiang X, Siegmund A, Connolly MD, Downing KH, Balsara NP, et al. Morphology and Proton Transport in Humidified Phosphonated Peptoid Block Copolymers. Macromolecules 2016;49(8):3083-3090. https://doi.org/10.1021/acs.macromol.6b00353
[29] Ye YS, Wang H, Bi SG, Xue Y, Xue ZG, Liao YG, et al. Enhanced ion transport in polymer–ionic liquid electrolytes containing ionic liquid-functionalised nanostructured carbon materials. Carbon 2015;86:86-97. https://doi.org/10.1016/j.carbon.2015.01.016
[30] Lobato J, Cañizares P, Rodrigo MA, Úbeda D, Pinar FJ. A novel titanium PBI-based composite membrane for high temperature PEMFCs. Journal of Membrane Science 2011;369(1):105-111. https://doi.org/10.1016/j.memsci.2010.11.051
[31] Arun Saco S, Thundil Karuppa Raj R, Karthikeyan P. A study on scaled up proton exchange membrane fuel cell with various flow channels for optimising power output by effective water management using numerical technique. Energy 2016;113:558-573. https://doi.org/10.1016/j.energy.2016.07.079
[32] Haase S, Moser M, Hirschfeld JA, Jozwiak K. Current density and catalyst-coated membrane resistance distribution of hydro-formed metallic bipolar plate fuel cell short stack with 250 cm2 active area. Journal of Power Sources 2016;301:251-260. https://doi.org/10.1016/j.jpowsour.2015.09.118
[33] Tayouo R, David G, Ameduri B, Roziere J, Roualdes S, David G. New Fluorinated Polymers Bearing Pendant Phosphonic Acid Groups. Proton Conducting Membranes for Fuel Cell. Int J Biol Macromol 2010;43(12):5269-5276. https://doi.org/10.1021/ma100703k





