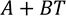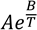PAM Review: Energy Science & Technology, Vol. 6
ISSN 2205-5231 | Published by UTS ePRESS | https://epress.lib.uts.edu.au/student-journals/index.php/PAMR/index
Characterisation of Molten Salts for Application in Molten Salt Reactors
Jake Barnes1, Ryan Coutts2, Toby Horne3 and Jesse Thai4
University of Technology Sydney, Faculty of Science, PO Box 123, Ultimo NSW 2017, Australia
1Jake.P.Barnes@student.uts.edu.au
2Ryan.Coutts@student.uts.edu.au
3Toby.M.Horne@student.uts.edu.au
4Jesse.Thai-1@student.uts.edu.au
DOI: https://doi.org/10.5130/pamr.v6i0.1546
Citation: Barnes, J., Coutts, R., Horne, T., and Thai, J. 2019. Characterisation of Molten Salts for Application in Molten Salt Reactors. PAM Review: Energy Science & Technology, 6, Article ID 1546. https://doi.org/10.5130/pamr.v6i0.1546
© 2019 by the author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License (https://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Vol. 6
Abstract
Molten Salt Reactors (MSRs) are one of the six Gen (IV) reactor designs chosen by the Generation IV International Forum for further development. A key area of concern for MSRs is the selection of molten salt composition. Parameters such as heat capacity, thermal conductivity, and viscosity are essential to consider when selecting a salt mixture for use as a coolant in an MSR. In this meta-study, the thermodynamic properties of a range of halide, carbonate and nitrate salts are compared. Using this data, an estimate is made for the usable energy density of each salt. This value in combination with the raw data is used to assess the viability of each salt for use in an MSR. It was found that fluoride salts are the most suitable. They tend to have high heat capacities and large thermal conductivities in relation to the other salts in this study. The 50-50 concentration of LiF-BeF2 had by far the highest usable energy density at 2.21 J/cm3K, however its extremely high viscosity, of 22.2 mPa.s, makes it unsuitable for use as a circulating coolant. LiF-NaF-BeF2 had the next highest usable energy density at 1.82 Jcm-3K-1. Without considering factors beyond thermodynamic properties, it was concluded that LiF-NaF-BeF2, would be the most suitable of the studied salts for use as an MSR coolant. Much of the experimental data in this field was obtained over 40 years ago, it is often of poor quality, lacking standardisation and with large error margins. An attempt has been made in this paper to compile this data and to standardise it to such a degree that salts can be reasonable compared.
Keywords
Molten Salt Reactor; FLiBe; FLiNaK; Heat Capacity; Carnot Efficiency
1. Introduction
1.1 Need for Nuclear Energy / CO2 Replacement
Global energy demands are increasing year on year this is happening with a simultaneous need to move away from fossil fuel-based energy production. This has led to a vast increase in renewable energy and a willingness to rediscover some technologies that have been left underdeveloped. A source of base load power that is CO2 free is a nuclear reactor [1]. The Generation IV international forum (GIF) comprised at the time of 9 countries created criteria for the future of nuclear development and agreed upon 6 reactor designs to pursue. These criteria are sustainability, safety, reliability, economic competitiveness, proliferation resistance and physical protection [2].
Nuclear fission is a highly dense energy source with a factor 107 greater energy density than chemical energy release. [3]. Proper utilisation of this energy density will allow us to replace CO2 producing energy systems. Nuclear plants have many properties that can complement renewable sources namely they can act as a stable base load power production that can be operated 24/7, they also have a small physical footprint so in areas where land is sparse, they can be implemented where other technologies cannot.
1.2 What is an MSR?
A molten salt reactor (MSR) is a type of nuclear fission reactor that can combine fissile and fertile material with halide salts commonly fluorides such as UF4, PuF3 and/or ThF4 to form a fluid [4]. MSR reactors have many benefits compared to the nuclear reactors currently in operation which have solid fuel cores and that are cooled by water under extreme pressures to achieve operating temperatures around 300 degrees Celsius.
Having a fluid fuel carrier allows for constant fuel level monitoring and operation without the need for shutting down the reactor. Molten salts have a high boiling point allowing for use in a reactor without the need for pressurisation and the ability to run at a much higher temperature allowing for greater conversion efficiency [5]. The ability to achieve higher fuel burn up resulting in far less waste and thus greater efficiency than current technology, self-regulating nuclear reaction due to the fluid nature of the fuel expanding during reaction as the fuel expands reaction slows down preventing the case of runaway fission [6].
Also due to their ability to run at higher temperatures 900K they can be used in H2 production at a far greater efficiency and lower cost than current electrolysis methods [7]. The waste heat from running these reactors could be further utilised to desalinate water providing another benefit whilst improving the overall efficiency of the process [8].
1.3 Development History
The start of extensive research into molten salt reactors commenced with the U.S. aircraft reactor experiment in support of the U.S. Aircraft Nuclear Propulsion program [9]. The decision to use molten fluoride salts for that program was the great stability of the salts, both to high temperatures and to radiation This ran successfully for 9 days [10]. Further research into molten salt reactor designs and system took place in the late 50s focusing on salt compositions and single or dual salt systems [11]. This resulted in in the construction on the molten salt reactor experiment, which started in 1962 and ran successfully for 5 years. Further design changes and modifications led to the design on the Molten Salt Breeder reactor [12] which is the design still largely unchanged for the Gen IV reactor.
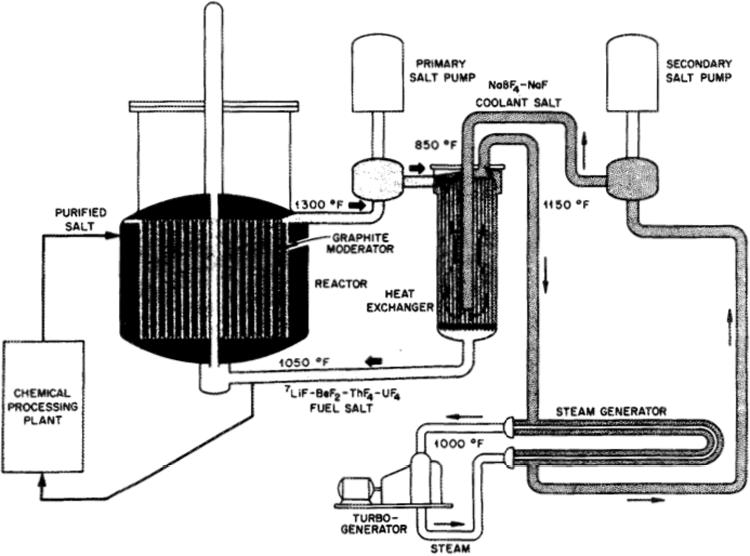
Figure 1 The 1970s Single Fluid, graphite moderated Molten Salt Breeder Reactor. Freeze plug and drainage system to dump tanks not shown [12].
1.4 Thorium Use and Waste Reduction
Thorium is three times more present in the Earth’s crust and is easily extracted when comparing to Uranium where we currently burn the isotope that is only fractions of a percent of total Uranium supplies [13]. Thorium is a naturally fertile material when thorium 232 absorbs a neutron and undergoes beta decay turning it into thorium 233 and then uranium 233 which is a fissile material [14]. One of the major drawbacks of current nuclear technologies is the spent waste. LWRs burn very little of the fissionable material in their solid cores and this waste will have to be stored for millennia. A molten salt reactor can take the waste material and use it to kick start a thorium fuel cycle as well as the fissile material from nuclear weapons [15] and use it to create power whilst simultaneously lowering the total amount of waste and the time that it has to be stored for [16].
1.5 Energy Utilisation
When fissile elements undergo radioactive decay one of the decay products is vast quantities of heat. To maintain operation of a nuclear reactor this decay heat has to be moderated and can be utilised to do useful work [17]. The choice of coolant is determined by the physical constraints of a system in this case being high temperatures and low to moderate volume. Molten halide salts are an ideal candidate due to their thermodynamic properties, their high heat capacity at constant pressure and volume allow for large amounts of thermal energy to be transported from the reactor to a generator as shown in Figure 1 [18].
The higher operational temperatures available now that molten salts are being used as the thermal moderator allow for new electricity generation systems to be used. A closed cycle gas turbine (CCGT), which operates on a Brayton cycle can achieve higher efficiency, simpler design and a smaller physical footprint than steam-based Rankine cycles systems [19].
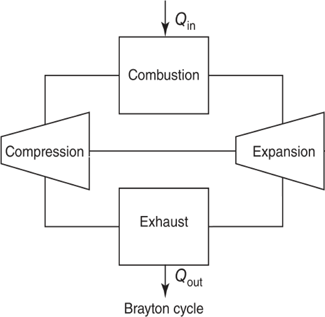
Figure 2 Flow diagram of a (CCGT) following a Brayton cycle [20].
1.6 Choice of Coolant Determination
The chemical and molar composition of the salt used in an MSR will be based on their thermodynamic properties e.g. heat capacity, melting point and viscosity at operating temperatures. We have selected an operating temperature of 977 K and will draw comparisons and judgments from this point to determine which salt/s are best suited to use in an operational MSR going forward. We are aiming to tie together studies from outset of molten salt research to present day and provide a framework of data to directly compare these salts.
2. Methods
2.1 Data Collection
For this meta-study, data was collected from SCOPUS, Web of Science, and Google Scholar, these databases were chosen as they were among the most comprehensive science-based databases available to the research team. The collected data was not limited to a timeframe due to the bulk of the experimental research in this area took place between the late 60s to early 80s. The scientific literature was obtained by searching for keywords such as “Molten salt”, “Molten Salt Reactors”, and the empirical formulas of salts and their properties of - composition, melting point, heat capacity, thermal conductivity, viscosity, density, and heat capacity. The salts researched were LiF-BeF2, NaF-BeF2, NaF-NaBeF4, NaF-ZrF4, LiF-NaF-BeF2, LiF-NaF-KF, LiF-Na2CO3-K2CO3, Li2CO3-Na2CO3-K2CO3, NaNO3-KNO3, NaNO3-NaNO2-KNO3, KCl-MgCl2; some independent salts varied in compositions (% moles).
Sources used in this study were required to satisfy the conditions of – having an emphasis on at least one salt, a detailed focus on molten salt reactors, and at least two thermophysical properties. Despite the majority of experimental research occurring between the late 60s to early 80s, a total of thirty-five scientific literatures within the past fifteen years successfully contributed to this study.
2.2 Data Analysis
For data comparison of researched salts, thermophysical properties were compared. The key parameters of melting point, heat capacity, viscosity, thermal conductivity, volumetric heat capacity, and Carnot efficiency were narrowed down and are tabulated in Table 1 and Appendix 1. Parameter relationships were explored graphically for Melting Point vs Heat Capacity, Viscosity vs Heat Capacity, Thermal Conductivity vs Heat Capacity, Thermal Conductivity vs Viscosity, and Volumetric Heat Capacity vs Carnot Efficiency. Parameters that are graphically illustrated required to provide quantitative value that can be deduced for further development of efficiency in molten salt reactors as a coolant and possible fuel source.
Standardisation of data in the units of Kelvin (K) for temperature and Joule per gram Kelvin (J/gK) for heat capacity was required for calculations of Carnot efficiency (Eq. 1); where is the critical temperature at which corrosion initiates, and is the melting point of the salt. Likewise, volumetric heat capacity (s) units are standardised and calculated by the product of specific heat capacity in J/gK and density (ρ) in g/cm3 (Eq. 2).


Table 1 Molten Salt Properties at Generalised Temperature (Unless Specified) Data acquired from multiple independent sources.
3. Results
| Salt | Composition (%mole) | Melting Point (K) | Max. Temp (K) | Heat Capacity (J/g.K) | Thermal Conductivity (W/m.K) | Viscosity (mPa.S) | Density (g/cm3) |
|---|---|---|---|---|---|---|---|
| LiF-BeF2 (A) | 69-31 | 778 [21] | 2.7196 [21] | 7.3 @600oC [21] | 1.88 [21] | ||
| LiF-BeF2 (B) | 66-34 | 727-731 [21] | 1703 [22] | 2.39 ±3% [23] | 1.1 ±10% [23] | 0.116, 3755 ±20% [23] | 2.413, -488×10-6 ±2% [23] |
| LiF-BeF2(C) | 50-50 | 623 [21] | 2.803 [21] | 22.2 @600 [21] | 2.18 [21] | ||
| NaF-BeF2 | 57-43 | 633 [11] | 2.18 [11] | 0.0346, 5164 [11] | 2.37, -360×10-6 [11] | ||
| NaF-NaBeF4 | 8-92 | 657 [24] | 973 [25] | 1.5 [24] | 0.66, -237×10-6 [24] | 0.0877, 2240 [24] | 2.4463, -711×10-6 [24] |
| NaF-ZrF4 | 50-50 | 783 [11] | 1623 [26] | 1.172 ±10% [23] | 0.49 ±15% [23] | 0.0709, 4168 ±10% [23] | 4.04, -930*10-6 ±5% [23] |
| LiF-NaF-BeF2 | 35-27-38 | 611 [11] | 2.47 [11] | 0.0388, 4738 [11] | 2.33, -410*10-6 [11] | ||
| LiF-NaF-KF | 46.5-11.5-42.0 | 727 [21] | 1843 [27] | 1.88 ±10% [23] | -0.0635, 1.4×10-3 [28] | 0.04, 4170 ±10% [23] | 2.579, -624×10-6 ±2% [23] |
| LiF-Na2CO3-K2CO3 | 50.9-19.8-29.3 | 695 [29] | 1224 [29] | 1.82 [28] | -0.135, 1.58×10-3 [28] | 0.0589, 4132.9 [28] | 2.31, -413×10-6 [28] |
| Li2CO3-Na2CO3-K2CO3 (A) | 46.2-30.0-23.9 | 670 [29] | 1148 [29] | 1.61 [28] | 0.336, 258×10-4 [28] | 0.0650, 4431.3 [28] | 2.27, -434×10-6 [28] |
| Li2CO3-Na2CO3-K2CO3 (B) | 43.5-31.5-25.0 | 673 [30] | 1073-1123 [30] | 1.415 [30] | 0.1012, 4017.17 [30] | ||
| NaNO3-KNO3 | 64-36 | 493 [30] | 862 [30] | 1.1 @600oC [30] | 0.55@400oC [30] | 3.26 @360oC [30] | |
| NaNO3-NaNO2-KNO3 | 7-49-44 | 415 [31] | 727-811 [32] | 1.56 [31] | 0.51-0.65 @400oC [30] | 3.16 @300oC [30] | 2.498-749.7×10-6T±2.0% [31] |
| KCl-MgCl2 | 67-33 | 708 [31] | 1073 [33] | 1.15 [31] | 0.532, -10-4±3.69×10-2 [34] | 24.24-0.0388T+1.784×10-5 ×T2±0.443 [34] | 2.0007, -457×10-6±1.5% [31] |
Thermal conductivity, viscosity and density are all dependent on temperature. Equations have been used to describe these properties where possible. Where an equation could not be found, the temperature at which the measurement was taken has been provided. Equations for thermal conductivity and density are linear and are of the form:
Viscosity equation (with the exception of KCl-MgCl2) can be represented by:
The constants and have been reported in the Table 1 using the format “”. Cells which use this notation have been bolded.
The thermal conductivity of LiF-BeF2 (FLiBe) varies very little with temperature and has a large uncertainty, the value of 1.1 is generally recommended as the best approximation for all temperatures between 750-1200 K [23]. It is worth noting that corrosiveness is also an important property of molten salts. An analysis of corrosion properties was considered beyond the scope of this report. For a detailed comparison of molten salt corrosion properties, see the dataset compiled by Raiman, S & Lee, S [35].
There are several points in Table 1 which are only valid for a single temperature. In three of these cases the valid temperature is 873K. This temperature was therefore chosen as a point of comparison in the following plots. For a detailed tabular comparison of these salts at a single temperature, see Table 3 in Appendix A.
Hastelloy N is an alloy developed by Oakridge for use with fluoride salts. It is rated for use up to 977K. Above this temperature corrosion becomes a significant issue [4]. In most cases, this temperature was used as an upper limit for the purpose of calculating efficiency. The Carnot efficiency in the above table is idealised, in practice, circulating molten salts could not be cooled all the way to their melting point. Some salts become unstable at a temperature below this limit. In these cases, the lower limit was used. It is likely that with advances in material science, this upper limit will increase. The lower temperature limit used to calculate the Carnot efficiency is highly idealised. In practice, circulating molten salts could not be cooled all the way to their melting point.
Volumetric heat capacity was calculated by multiplying specific heat capacity with density. This is an important parameter when assessing molten salts, as it can be used to determine the reactor size required to store a specified quantity of heat. The product of the Carnot efficiency and the heat capacity tells us how much of this stored heat is theoretically usable.
Heat capacity and melting point are important characteristics to focus on when identifying an ideal molten salt for an MSR. The heat capacity is the amount of energy the salt can carry around the system whereas a lower melting point is important to determine what temperature range the MSR can operate at. The relationship between the two have been given in Figure 3 below.
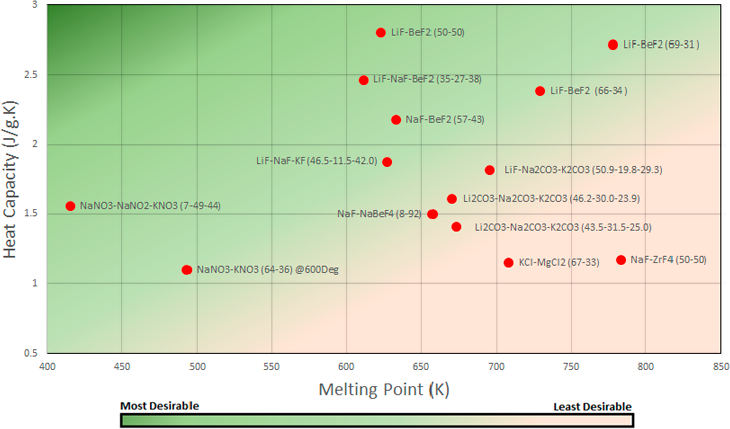
Figure 3 Melting Point vs Heat Capacity - Colour gradient included to optimum characteristics which in this graph is lower melting point and higher heat capacity
The most desirable salt should have a high heat capacity so more energy is transferred through the system and to have a lower melting point. The fluoride salt compositions such as LiF-BeF₂ and NaF-BeF₂ tend to have a higher heat capacity of 2.1-3 J/g.K but as a consequence have a higher melting point. The Sodium-Potassium salt compositions have a lower melting point but are significantly lower in heat capacity. For a MSR that operating at approximately 973K the most ideal molten salts in terms of heat capacity are LiF-BeF₂ (50-50) and LiF-BeF₂ (69-31) with 2.803 J/g.K and 2.7196 J/g.K respectively.
Another important characteristic to consider when selecting a molten salt for a reactor is the salt viscosity. The viscosity delegates how easily the salt fluid passes through the MSR system. The smaller the viscosity the lower amount of energy is required to pump the liquid salt around as well as lowering the chance of any build ups of blockages. Viscosity for various salts have been found at 873K and compared with heat capacity in Figure 4:
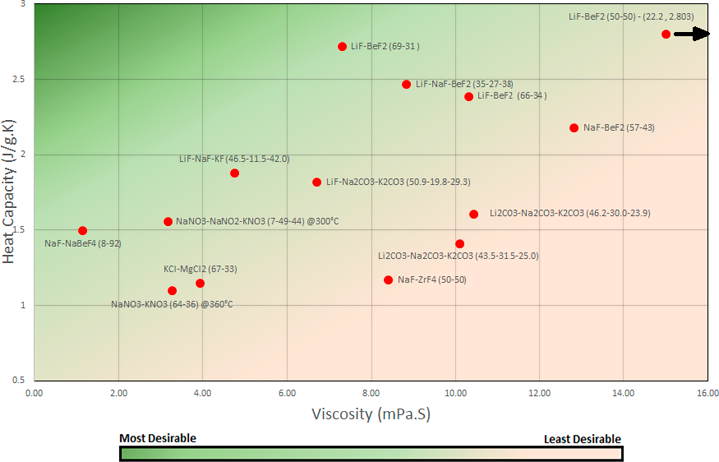
Figure 4 Viscosity (@873K) vs Heat Capacity - Where optimum characteristics are low viscosity and high heat capacity shown through the colour gradient.
The lithium - fluoride salt compositions have a well-balanced combination of heat capacity and viscosity compared to the other compositions of salt; with the exception of LiF-BeF₂ (50-50) which is an outlier with a very high viscosity level of 22.2 mPa.S. Although there is no single optimum salt composition that accounts for both heat capacity and viscosity, certain salts will be more efficient for the design of the salt transporting systems in MSR’s.
The most ideal salts that were compared are: LiF-BeF₂ (69-31) with viscosity of 7.3 mPa.S and heat capacity of 2.72J/g.K and LiF-NaF-KF (46.5-11.5-42.0) with viscosity of 4.75 mPa.S and a heat capacity of 1.88 J/g.K.
A comparison for which salts composition can conduct a larger amount of heat is important in how efficient an MSR operates, the higher the thermal conductivity value is more desirable when selecting molten salts. Due to the data found a comparison of salts are made at 673K and 873K. A graph of thermal conductivity (673K) is compared with heat capacity in Figure 5 below:

Figure 5 Thermal Conductivity (673K) vs Heat Capacity - Optimum thermal conductivity and heat capacity shown as the higher values of each parameter represented in the colour gradient.
From the available data it is clear that there are two salt compositions that are most optimum; these include LiF-Na₂CO₃-K₂CO₃ (50.9-19.8-29.3) with a heat capacity of 1.82 J/g.K and thermal conductivity 0.93 W/m.K, and LiF-NaF-KF (46.5-11.5-42.0) with 1.88 J/g.K and 0.88W/m.K. all other salts have a significantly lower heat capacity and thermal conductivity.
A comparison of thermal conductivity (873K) and heat capacity are compared in Figure 6. Similar to the comparison of thermal conductivity and heat capacity in Figure 5 there is a significant gap between three optimum salts and the remainder of the compositions. LiF-NaF-KF (46.5-11.5-42.0) and LiF-Na₂CO₃-K₂CO₃ (50.9-19.8-29.3) have a moderately high heat capacity of ≈ 1.85 J/g.K with a higher thermal conductivity of 1.15-1.25 W/m.K. LiF-BeF₂ (66-34) has a larger heat capacity of 2.39 J/g.K compare with all other salts but a lower thermal conductivity of 1.1 W/m.K. Another trend found from comparing Figure 5 and Figure 6 are that as you increase the temperature of the salt the higher the thermal conductivity will be, even more to the salts that already had a higher value.
Volumetric heat capacity is how much energy is stored as heat in the molten salts. Compare this characteristic with the Carnot efficiency shows us how much of the of the stored energy is the salt can be theoretically used. An optimum salt composition is one that has a high Carnot efficiency and a high volumetric heat capacity. Volumetric heat capacity and Carnot efficiency are graphed in Figure 7.
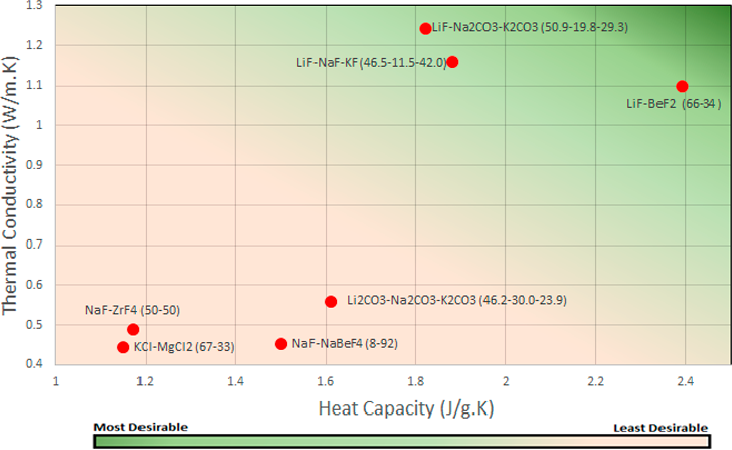
Figure 6 Thermal Conductivity (873K) Vs Heat Capacity - Most desirable thermal conductivity and heat capacity are towards the upper right side of the graph as shown through the colour gradient.
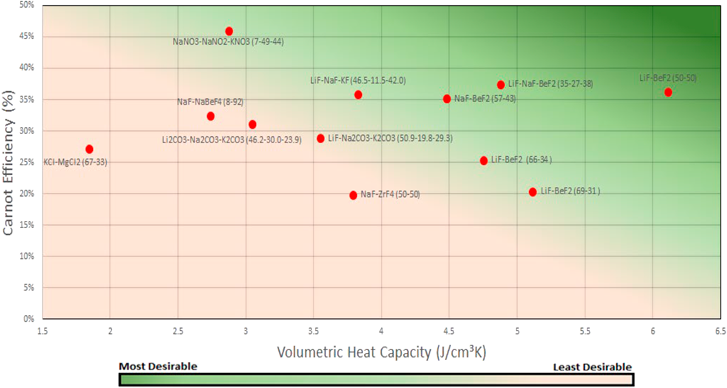
Figure 7 Volumetric Heat Capacity Vs Carnot Efficiency - High Carnot efficiency and higher volumetric heat capacity are ideal when comparing molten salts as expressed through the colour gradient.
The salt composition that has the highest Carnot efficiency is NaNO₃-NaNO₂-KNOB (7-49-44) with an efficiency at 46% but has a relatively low volumetric heat capacity of 1.56 J/cm³.K. The most optimum molten salt composition is the LiF-BeF(50-50) with volumetric heat capacity of 6.1 J/cm³.K and Carnot efficiency of 36%. The other notable salts that had a Carnot efficiency of >35% and volumetric heat capacity >4.4 J/cm³.K are NaF-BeF2 (57-43) and LiF-NaF-BeF (35-27-38). The Lithium-Fluoride and Sodium Fluoride salts are found to hold the most heat energy per volume but vary in their efficiency due to slight changes in the salt composition.
4. Discussion
4.1 Research Limitations
A large amount of data was available for this study; however, the quality of this data was often questionable. Although many of the articles cited in this paper are from post 2010, the experimental data referenced in these articles often came from the late 50s to early 70s. The review written by Romatoski RR and Hu LW [23] does an excellent job compiling the research undertaken over the last 50 decades, in regard to thermodynamic parameters of molten fluoride salts. They found that properties like heat capacity, thermal conductivity and viscosity often had uncertainties in the range of 10-20%, density tended to have errors in range of 2-5%. When available, these error margins have been included in Table 1. Many papers though did not list the errors associated with their data. When interpreting the results, it is important to consider that the errors could be somewhere in this vicinity.
Another limitation with these results is the lack of standardisation among papers. Studies were often conducted at different temperatures, and compositions. For the vast majority of temperature dependant variables, generalised trends have been found; however, some potentially interesting salts had to be excluded from this study, simply because there was either insufficient data, or because the data that was available was only valid for a specific temperature.
4.2 Data Analysis
Through analysis on the data it has been clear that the molten salts that have most desirable characteristics are the fluoride salts. The Lithium-Fluoride compositions have the higher heat capacities for MSR that are to operate at temperatures > 600K (Figure 3.) but have a higher viscosity which can cause possible issues to the practical operation of MSR systems (Figure 4.). The Lithium-Fluoride salt compositions also show excellent thermal conductivity when compared to the combinations such as Sodium-Fluoride and Potassium, it was also found that with an increase in temperature the thermal conductivity of Fluoride compositions increased more than the other salt compositions (Figure 5 & Figure 6.) although more research is required to quantify this trend. Lithium-Fluoride and Sodium-Fluoride salts have a desirable volumetric heat capacity while maintaining a relatively high Carnot efficiency where similar compositions without the fluoride have similar or higher thermal efficiency but a lower volumetric heat capacity. Taking the product of the efficiency and volumetric heat capacity allowed us to find which salts will provide the optimum theoretical heat transfer in the system. In Table 2, it is interesting to observe that the two Nitrate salts had the highest Carnot efficiencies. This is largely because they are unrestricted by material limitations and can therefore be operated across the entire stable liquid temperature range. It is important to note that with advances in materials technology the Nitrate salts will likely be overtaken, as technological advances allow reactors to be operated at higher temperatures. When the Carnot efficiency is used to calculate the usable energy density, the Fluoride salts again come out on top. The 50-50 concentration of LiF-BeF2 has by far the highest value as 1.01 J/cm3K, however as shown in Figure 3, it’s extremely high viscosity would make it very challenging to use as a coolant. LiF-NaF-BeF2 has the next highest value at 0.86 J/cm3K. Looking at Figures 2-6, LiF-NaF-BeF2 consistently appears on the more desirable end of the spectrum, making this salt an ideal coolant.
5. Conclusions
Based solely on the thermodynamic properties studied in this paper, the Fluoride salts seem to be the ideal candidates for use as a coolant in MSRs. LiF-NaF-BeF2 specifically had the largest usable volumetric heat capacity, a value calculated by taking the product of the Carnot efficiency, the specific heat capacity and the density. Unfortunately, no data on the thermal conductivity of LiF-NaF-BeF2 could be obtained, however given the high thermal conductivities of the other mixtures containing LiF and/or BeF2, it seems likely that LiF-NaF-BeF2, would follow this trend. As this technology develops, we predict that efficiency will become less dependent on the limiting temperature imposed by the vessel material (as is currently the case). A more significant factor could be the maximum stable temperature limit of the salts. In general, the values reported for thermal conductivity were quite poor, many studies reported large uncertainties in the range of ±10-20%, and for many salts, no information regarding thermal conductivity could be found. This field of study would benefit greatly by having some standardised testing conducted across a wide range of molten salts on both thermal conductivity and viscosity, which was also regularly cited with large error margins.
References
1. Mathieu L, Heuer D, Brissot R, Le Brun C, Liatard E, Loiseaux J, et al. The Thorium Molten Salt Reactor : Moving On from the MSBR. 2005.
2. OECD/NEA. Technology Roadmap Update for Generation IV Nuclear Energy Systems: Preparing Today for Tomorrow’s Energy Needs 2014:7.
3. Le Brun C. Molten salts and nuclear energy production. J Nucl Mater 2007;360:1–5. doi:10.1016/j.jnucmat.2006.08.017.
4. LeBlanc D. Molten salt reactors: A new beginning for an old idea. Nucl Eng Des 2010;240:1644–56. doi:10.1016/j.nucengdes.2009.12.033.
5. Rosenthal MW, Kasten PR, Briggs RB. Molten-Salt Reactors—History, Status, and Potential. Nucl Appl Technol 2017;8:107–17. doi:10.13182/nt70-a28619.
6. Uhlíř J, Beneš O, Kloosterman JL, Yoshioka R, Delpech S, Zhimin D, et al. The molten salt reactor (MSR) in generation IV: Overview and perspectives. Prog Nucl Energy 2014;77:308–19. doi:10.1016/j.pnucene.2014.02.014.
7. Forsberg CW. Hydrogen, nuclear energy, and the advanced high-temperature reactor. Int J Hydrogen Energy 2003;28:1073–81. doi:10.1016/S0360-3199(02)00232-X.
8. Dardour S, Nisan S, Charbit F. Utilisation of waste heat from GT-MHR and PBMR reactors for nuclear desalination. DESALINATION 2007;205:254–68. doi:10.1016/j.desal.2006.03.554.
9. Bettis ES, Schroeder RW, Cristy GA, Savage HW, Affel RG, Hemphill LF. The Aircraft Reactor Experiment—Design and Construction. Nucl Sci Eng 1957;2:804–25. doi:10.13182/NSE57-A35495.
10. MacPherson HG. The Molten Salt Reactor Adventure. Nucl Sci Eng 2016;90:374–80. doi:10.13182/nse90-374.
11. MacPherson HG. Chemical Aspects of Molten-Fluoride-Salt Reactor Fuels. Addison-Wesley, 1958, p. 969.
12. Rosenthal MW, Haubenreich PN, Briggs RB. The Development Status of Molten-Salt Breeder Reactors, Oak Ridge National Laboratory, ORNL-4812. Ornl-4812 1972.
13. Acta C, Ireland X. Abundance of chemical elements in the continental crust : a new table 1964;28.
14. Hargraves, Robert; Moir R. Liquid Fluoride Thorium Reactors_Am Scientist 2010.pdf. Am Sci 2010;98:304–13.
15. Gat U, Engel JR, Dodds HL. Molten-Salt Reactors for Burning Dismantled Weapons Fuel. Nucl Technol 1992;100:390–4. doi:10.13182/NT92-A34733.
16. Kormilitsyn M V., Khokhlov VA, Ignatiev V V., Subbotin VG, Afonichkin VK, Surenkov AI, et al. Molten-salt reactors: new possibilities, problems and solutions. At Energy 2012;112:157–65. doi:10.1007/s10512-012-9537-2.
17. Garvey G. Nuclear Power. Energy, Ecol Econ 2015:135–56. doi:10.1007/978-1-349-02421-6_7.
18. HançerlioğullarI A. Thermodynamics properties of molten salt technology assessment for new generation fusion reactors. J Fusion Energy 2014;33:463–70. doi:10.1007/s10894-014-9694-5.
19. Olumayegun O, Wang M, Kelsall G. Closed-cycle gas turbine for power generation: A state-of-the-art review. Fuel 2016;180:694–717. doi:10.1016/j.fuel.2016.04.074.
20. Bunce RH. Gas turbines. Energy Conversion, Second Ed 2017:209–22. doi:10.1201/9781315374192.
21. Bahri CNACZ, Al-Areqi WM, Ruf MIFM, Majid AA. Characteristic of molten fluoride salt system LiF-BeF2 (Flibe) and LiF-NaF-KF (flinak) as coolant and fuel carrier in molten salt reactor (MSR). AIP Conf Proc 2017;1799. doi:10.1063/1.4972932.
22. Ying D, Yang H, Lyu H, Tan Y, Jing F, Li L, et al. Activation of FLiBe coolant in the molten salt reactor. Ann Nucl Energy 2019;129:62–6. doi:10.1016/j.anucene.2019.01.038.
23. Romatoski RR, Hu LW. Fluoride salt coolant properties for nuclear reactor applications: A review. Ann Nucl Energy 2017;109:635–47. doi:10.1016/j.anucene.2017.05.036.
24. Beneš O, Konings RJM. Thermodynamic properties and phase diagrams of fluoride salts for nuclear applications. J Fluor Chem 2009;130:22–9. doi:10.1016/j.jfluchem.2008.07.014.
25. Mohan G, Venkataraman MB, Coventry J. Sensible energy storage options for concentrating solar power plants operating above 600 °C. Renew Sustain Energy Rev 2019;107:319–37. doi:10.1016/j.rser.2019.01.062.
26. Williams DF. Assessment of Candidate Molten Salt Coolants for the NGNP/NHI Heat-Transfer Loop. 2006. doi:ORNL/TM-2006/69.
27. Patel NS, Pavlík V, Kubíková B, Nosko M, Danielik V, Boča M. Corrosion behaviour of Ni-based superalloys in molten FLiNaK salts. Corros Eng Sci Technol 2019;54:46–53. doi:10.1080/1478422X.2018.1525829.
28. An XH, Cheng JH, Su T, Zhang P. Determination of thermal physical properties of alkali fluoride/carbonate eutectic molten salt. AIP Conf Proc 2017;1850. doi:10.1063/1.4984415.
29. Reddy RG. Novel Molten Salts Thermal Energy Storage for Concentrating Solar Power Generation 2013:1–27. doi:10.2172/1111584.
30. Vignarooban K, Xu X, Arvay A, Hsu K, Kannan AM. Heat transfer fluids for concentrating solar power systems - A review. Appl Energy 2015;146:383–96. doi:10.1016/j.apenergy.2015.01.125.
31. Manohar S. Sohal, Matthias A. Ebner, Piyush Sabhar. Engineering Database of Liquid Salt Thermophysical 2013. doi:10.2172/1086824.
32. Villada C, Bonk A, Bauer T, Bolívar F. High-temperature stability of nitrate/nitrite molten salt mixtures under different atmospheres. Appl Energy 2018;226:107–15. doi:10.1016/j.apenergy.2018.05.101.
33. Anderson NA, Sabharwall P. Molten Salt Mixture Properties (KF-ZrF 4 and KCl-MgCl 2 ) for Use in RELAP5-3D for High-Temperature Reactor Application . Nucl Technol 2017;178:335–40. doi:10.13182/nt12-a13598.
34. Xu X, Wang X, Li P, Li Y, Hao Q, Xiao B, et al. Experimental Test of Properties of KCl–MgCl 2 Eutectic Molten Salt for Heat Transfer and Thermal Storage Fluid in Concentrated Solar Power Systems . J Sol Energy Eng 2018;140:051011. doi:10.1115/1.4040065.
35. Raiman SS, Lee S. Aggregation and data analysis of corrosion studies in molten chloride and fluoride salts. J Nucl Mater 2018;511:523–35. doi:10.1016/j.jnucmat.2018.07.036.