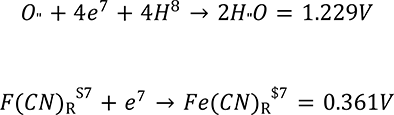PAM Review: Energy Science & Technology, Vol. 5, 2018
ISSN 2205-5231 | Published by UTS ePRESS | https://epress.lib.uts.edu.au/student-journals/index.php/PAMR/index
Wastewater and Mixed Microbial Consortia: a metastudy analysis of Optimal Microbial Fuel Cell configuration
Jonathon Ryan1,*, Hayden Ferral-Smith2 and Joshua Wilson3
University of Technology Sydney, Faculty of Science, PO Box 123, Ultimo NSW 2017, Australia
1 jonathon.j.ryan@student.uts.edu.au
2 hayden.ferral-smith@student.uts.edu.au
3 joshua.wilson-2@student.uts.edu.au
Corresponding author: Jonathon Ryan, University of Technology Sydney, Faculty of Science, PO Box 123, Ultimo NSW 2017, Australia; jonathon.j.ryan@student.uts.edu.au
DOI: http://dx.doi.org/10.5130/pamr.v5i0.1496
Citation: Ryan, J. et al. 2018 . Wastewater and Mixed Microbial Consortia: a metastudy analysis of Optimal Microbial Fuel Cell configuration. PAM Review: Energy Science & Technology, Vol. 5, pp. 22-36. http://dx.doi.org/10.5130/pamr.v5i0.1496
© 2018 by the author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License (https://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Abstract
Microbial Fuel Cells (MFCs) are an area of increasing research for use as an alternative energy source, due to their ability to produce electricity while simultaneously treating organic waste. This meta-study determines the optimal MFC configuration for electricity production, through consideration of the biocatalyst and substrate used. This study focuses primarily on comparing the use of mixed microbial consortia to pure strains of biocatalyst, and the use of waste water in contrast to simple substrates such as; acetate, glucose, and lactate. The use of algae as a substrate, and as a biocatalyst, is also investigated. In this study, only single and dual chamber MFCs are compared, and power density standardised to anode surface area (mW/m2) is used as a metric to facilitate the comparison of different experimental setups. This meta-study shows that dual chamber MFCs, using simple substrates, when catalysed by mixed culture biocatalysts, produce greater power densities, than algae, and complex substrates, with average power densities of 280, 70 and 30 (mW/m2) observed respectively. In single chamber MFC configurations, mixed culture biocatalysts have been observed to yield approximately double the power output of pure culture biocatalysts.
Keywords
Microbial Fuel Cell; MFC; algae; biocatalyst; wastewater; mixed microbial consortia; dual chamber; single chamber
| Glossary | |
|---|---|
| MFC | Microbial Fuel Cell, device that converts the chemical energy of organic molecules into electrical energy via redox reaction. |
| Power Density | mW/m2, power output standardised against anode surface area, to facilitate comparison. |
| Substrate | Fuel input to the MFC. An organic molecule oxidised by a biocatalyst at the anode. |
| Biocatalyst | Microorganism used to liberate electrons from the substrate, commonly bacteria. |
| PEM | Proton Exchange Membrane, a semi-permeable membrane separating the anode and cathode chambers in a dual chamber MFC. Facilitates the transfer of ions between chambers, but blocks molecules. |
| Exo-electrogens | Microbes that possess the ability to transfer electrons from substrate material to anode surface, occurs through various biological mechanisms. |
| Microbial Consortia | A community of multiple microbes, often undefined, as that harvested from wastewater, or defined, such as that synthesised. |
Introduction
A microbial fuel cell (MFC) is a device that directly converts the chemical energy of an organic substrate into electrical energy via redox reaction. MFCs most commonly consists of separate anode and cathode chambers separated by a proton exchange membrane. Electrons are liberated from the substrate through an oxidation reaction, and then flow via the anode, through a resistive load and to the cathode. At the cathode, the electrons are then used to reduce a catholyte, most commonly oxygen, completing the electrochemical redox reaction [1]. For example, the oxidation of acetic acid in the anode chamber is shown in Equation 1, followed by the general cathodic reduction half Equation of oxygen, shown in Equation 2 [2].
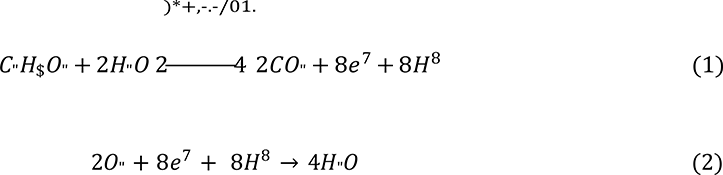
Biocatalysts in MFCs
An MFC is characterised by the fact that an active microorganism is used to catalyse the oxidation reaction in the anode chamber. As such, the anode chamber must remain an anaerobic environment to prevent the microbes from photosynthesising, which would inhibit electricity production [2]. The PEM allows protons to migrate from the anode chamber, to the cathode chamber, while preventing molecules such as oxygen from entering the anode chamber [4].
Once liberated from the substrate material, electrons must be conveyed to the anode surface in order for electricity to be conducted. Electron mediators may be used for this purpose, however these chemicals are consumable, and often toxic, so will not be considered in this paper [4]. Rather, some microbes, referred to as exo-electrogens, possess the ability to transfer electrons directly from the substrate to the anode surface. There are a number of known biological mechanisms through which these exo- electrogens can conduct electrons to the surface of the anode, such as; the formation of so called ‘nanowires’, the excretion of non-toxic mediators, and direct conduction through the formation of a ‘biofilm’ on the surface of the anode [5,6]. Such biological mechanisms are, however, not the topic of this meta-study, and as such, are not investigated in any further detail. These mechanisms must be highlighted however, as it is reasoned that electron conduction may be enhanced by the simultaneous presence of a combination of these electron conduction mechanisms. This study will therefore investigate the performance of mixed microbial consortia in MFCs.
Although pure microbial cultures may be used to catalyse the anodic oxidation reaction, it is often easier to obtain mixed microbial consortia. This mixed microbial consortium may be undefined, such as that harvested from waste water, or a synthesised and therefore defined consortia [7]. Literature has shown that microbes are most often sourced from readily available, natural sources such as; wastewater (household, industrial & agricultural) [3,4, 8-12], landfill leachate [13], and manure [14]. Pure microbes are also readily used [9].
MFC Configuration
Research has shown that dual chamber MFCs are the most commonly investigated MFC configuration. The dual chamber MFC is characterised by the two separate anode and cathode chambers. An overview of a typical dual chamber MFC is shown in Figure 1 below.
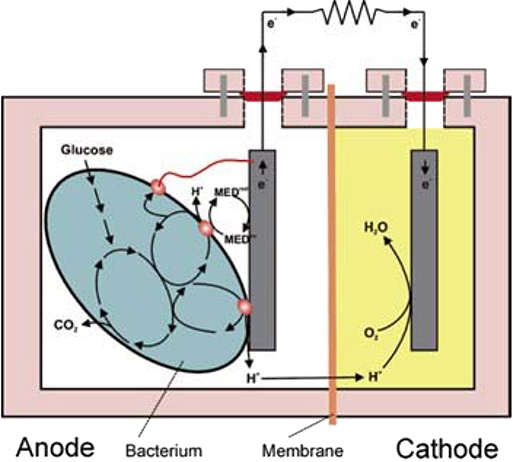
Figure 1 The fundamental design of a dual chamber MFC. Substrate and microbes are shown in the anode chamber, a proton exchange membrane separates the cathode chamber. Electrons are liberated from the substrate in the anode chamber via oxidation reaction, flow from anode to cathode via a resistive load, and are reduced at the cathode chamber, completing the redox reaction [1].
Other MFC configurations such as single chamber MFCs are also regularly referenced in literature. Single chamber MFC’s contain one common chamber shared by both the anode and cathode as shown in Figure 2. The lack of a PEM is the major distinguishing factor between the two configurations. These types of MFCs generally contain an air exposed-cathode, however this is not necessary to the design; for the purpose of this study however we have limited our single chamber designs to this air-exposed cathode style [15].
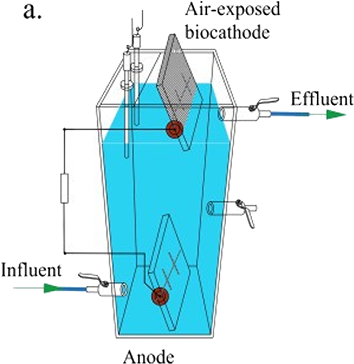
Figure 2 Single chamber configuration showing anode and cathode within the same chamber. No separation between each area of the chamber and the presence of an air-exposed cathode as shown [15].
Algal MFCs
The use of algae in MFCs is the subject of much research currently, due to its diverse use, and the range of benefits the algae provide [4, 7-8, 14, 16-19]. Algal biomass may be introduced to the anode chamber, to provide a fuel source, or alternatively, algae may be photosynthesised in the cathode chamber to sustainably produce oxygen for the reduction half reaction [7, 17, 19] Some more complex algal MFCs employ an external ‘photo bioreactor’ to externally photosynthesise the algae, producing oxygen to catalyse the cathode chamber reduction reaction, and using the waste algae biomass as a substrate in the anode chamber [14, 17]. Algal MFCs are hence increasingly researched, due to their ability to provide a self-sustaining MFC solution. Figure 3, shown below describes a dual chamber algal MFC, where algae is photosynthesised in the cathode chamber, and algal biomass is introduced to the anode chamber.
The most commonly used algae strain identified in literature is, C.vulgaris [4, 7-8, 14]. Other algae used in MFCs include U.lactuca [8] and blue-green algae [7, 12].
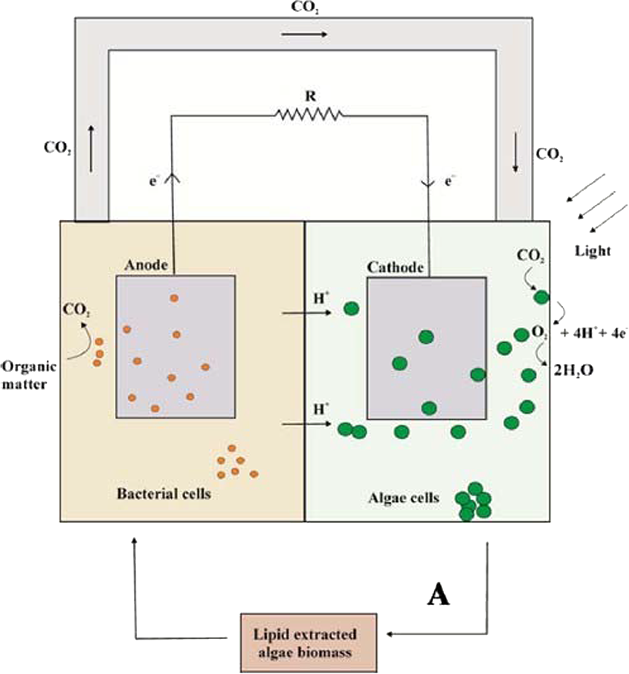
Figure 3 Typical dual chamber, algal MFC design. Algae is used in both anode and cathode chambers. Algae may be used as a substrate in the anode chamber, or photosynthesised in the cathode chamber to produce oxygen for the cathode reduction reaction [14].
Thermodynamics of MFCs
The simplest way to evaluate the thermodynamics of an MFC, is in terms of the electromotive force (EMF). The net EMF of an MFC is defined as the potential difference between the cathode and anode electrodes, Equation 3. That is, the difference in reduction potential between the cathode and anode redox reactions. The work done by the MFC is therefore equivalent to the product of net EMF and the electron charge transferred from the anode to the cathode Equation 4 [1].

From Equations 3 and 4, the net work done by the MFC is directly attributed to the anodic half reaction. With the Coulombic charge and the anode EMF directly attributed to the substrate used in the MFC, it is obvious that the choice of substrate will heavily impact the power output of the MFC.
The cathode reduction potential, however, also impacts the net work done by the MFC. As such, identical anode conditions coupled with different cathode conditions will yield different MFC power output. Thus, to ensure the results presented in this metastudy are comparable, cathode conditions will be kept identical in terms of EMF.
As mentioned, oxygen is commonly reduced at the cathode. Ferricyanide however, is also commonly used as an electron acceptor in MFCs. [1, 9, 20-22]. The reduction potential of oxygen compared to ferricyanide is shown below;
The availability of oxygen cathode MFC experimental setups is sufficient to allow the restriction of this metastudy analysis to exclude ferricyanide. Additionally, ferricyanide is toxic and hence not used in many of the algal MFCs studied [4]. However, a summary of this literature search is provided as an appendix, including those studies which have used artificial electron mediators such as ferricyanide.
With the cathode conditions kept constant, the anode conditions can then be varied, to determine the effects of MFC substrate and biocatalyst. While simultaneously logging net EMF and current, a variable resistor connected between the anode and cathode electrodes, can be used to determine the peak power output by the MFC, this process is often referred to as polarisation [23]. Since the surface area of the anode will limit the rate at which electrons can flow to the cathode, and hence the work the MFC can do, MFC power output must be standardised against anode surface area, to allow the valid comparison of different MFC setups [1]. Thus, the most universal comparison metric is maximum power output per unit area of anode surface, Equation 5;

More comprehensive analysis of the thermodynamics of an MFC, shows that the maximum work done by the MFC may be related to the free energy change of the net reaction. The reaction is evaluated using Gibbs free energy, as it is more likely the MFC will remain at constant temperature and pressure, rather than at constant volume [24].

For a reversible chemical reaction, such as that occurring in an MFC, the Gibbs free energy is given by;
Where ∆𝐺] is the Gibbs free energy under standard conditions, calculated from the known energies of formation of the substrates introduced to the MFC, and Π is the reaction quotient, which is equivalent to the activities of the products divided by that of the reactants [1, 24-25].
We also know that for isobaric and isothermal processes, the Gibbs function states that the change in Gibbs free energy is equivalent to the difference between the change in enthalpy, and the amount of heat produced by the reaction, shown in Equation 8 [24];

This analysis is particularly useful, as it shows the effects of temperature change on the MFC net voltage. The relationship shown in Equation 9 is yielded by taking the partial derivative of Equation 8, with respect to pressure. Note, as above, VNET refers to MFC net potential, not volume.

Since the chemical reactions occurring in the MFC are reversible, the Entropy change of the isolated MFC is therefore negative. As such, from Equation 9, the net potential of the MFC will decrease if temperature is increased [24].
Factors affecting the efficiency of MFCs
The observed potential difference across the MFC anode and cathode is consistently lower than that theoretically shown above. The three primary causes for these losses can be attributed to; internal resistance, activation energy and bacterial metabolism [1]. In dual chamber MFCs the PEM is the greatest contributor of internal resistance. Losses due to activation energy refer to the energy used in transporting electrons from the biocatalyst to the anode surface. Similarly, bacterial metabolic losses refer to that energy consumed by the bacteria in metabolising the substrate material. As such, physical constraints and current technology limit the maximum MFC power output [1, 6].
Methods
The research databases Science Direct, Google Scholar and Scopus were used to find the research articles referenced in this meta study. Initially “Microbial Fuel Cells (MFC)” was used to form the basis of our searches, and publications were restricted to those articles published between 2010-2018 in order to ensure the most recent research was investigated. Searches were then restricted via the addition of key terms such as “Algae,” “Biocatalyst,” “Substrate,” and “Fuel Source” as these were observed to be the most influential factors to MFC performance. As we began to collect data and identify the key types of substrates and biocatalysts we would study, we further refined our search terms and removed publication year restrictions. At this point we focused on obtaining quality publications as defined by the number of citations.
Energy output in Power Density was determined to be the most universal and comparable value across the papers researched. If mW/m2 was not published in the paper, we would calculate this value by standardising power output to the anode surface area. This allowed us to compare values across multiple papers much easier through this standardised value. With the data collected from all of our sources we sought to investigate any potential relationships between power density and different variables. Variables such as substrate, configuration (single vs double chamber), biocatalyst, and even the size of different working models were all researched and compared. A tabulated summary of all our research is provided as an appendix for reference.
Graphs were then created once enough data had been found, to make it easier to determine any trends in the results. Large outliers were identified within our data that we could not explain. As a result, these particular papers were re-analysed to determine any distinguishing factors or trends that could have caused this. It was discovered that in all of these setups, the presence of Ferricyanide was a common recurring substance. Ferricyanide changes the oxidation reaction that occurs at the cathode, thus to keep variables constant these papers and results were omitted to ensure a more consistent and valid comparison.
From these finally selected results, two styles of graphs were produced to show the results. Box and whisker and scatter plot graphs were selected to display these results as we believed these gave the best representation of our data. Initially 3 box and whisker graphs were chosen to display our results, however due to the inaccuracy of this style, these were later changed to scatter-plots to give a more transparent view of the results. These graphs more clearly show the differences between each substrate and biocatalyst used.
Results and Discussion
A comprehensive summary of all results obtained from this literature search are provided as an appendix, presented in a table format. This table also includes all experimental setups that were later found to contain artificial electron mediators in the cathode chamber. MFC configurations containing electron mediators such as ferricyanide, although not analysed, are still included in this appendix, for future reference.
Analysis of 20 individual, first hand investigations, comprising 54 different experiments, have yielded the following results. This meta-study has focused on investigating relationships between maximum power output and; MFC configuration, substrate and biocatalyst. These findings have been analysed, and are summarised in the graphs produced below;
Biocatalysts in MFCs
The primary purpose of this meta-study with regards to MFC biocatalysts, is to investigate the differences between pure, and mixed culture biocatalysts, to ideally make a recommendation as to how the biocatalyst should be implemented in an MFC to maximise power density. Figures 4-6 are presented below, to highlight the differences observed between single and mixed culture biocatalysts in single and dual chamber MFC configurations. Power Density values for Rhodopseudomonas Palustris were extrapolated from the data provided in paper 26, Wastewater Treatment Plant 8 and Wetland Consortium, values were calculated from paper 27.
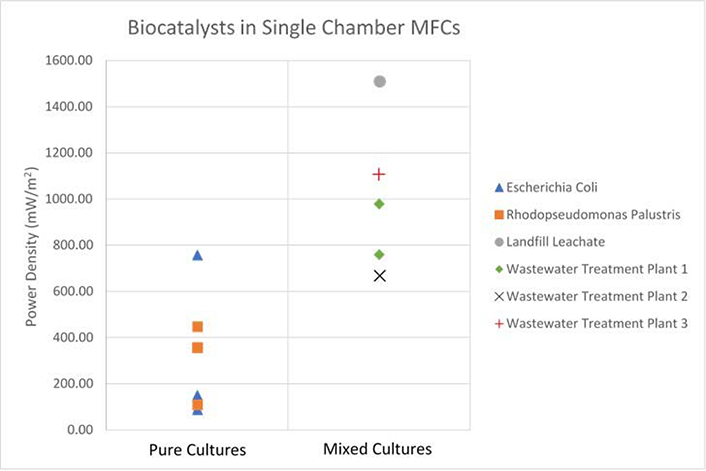
Figure 4 Single chamber MFC experimental setups, showing maximum power density for pure and mixed microbial cultures. 12 data points are presented. Note the trend that mixed culture biocatalysts yield approximately double the average power output of pure cultures, in single chamber configurations. Note, Power Density values for Rhodopseudomonas Palustris were extrapolated from the data provided in paper 26, Wastewater Treatment Plant 8 and Wetland Consortium, values were calculated from paper 27.
From Figure 4, the collected data suggests that mixed culture biocatalysts can achieve approximately double the power output of pure culture biocatalysts in single chamber MFCs. Data collected for dual chamber configurations, as shown in Figure 5, does not present a clear trend. With the exception of one outlier, all pure culture, dual chamber setups yield power densities between 3 and 50 mW/m2, while mixed culture MFCs yield a much broader spread of data, ranging from as low as 13.5 mW/m2 in one experiment, to as high as 283 mW/m2 in another.
The diverse spread of data shown in Figure 5 must be attributed to differences in the substrate used. This literature search, although quite comprehensive, is limited in that experimental setups are very different between publications. It was not possible on all occasions, to restrict analysis to one single variable. As such, and as in this case, to compare biocatalysts in dual chamber MFCs, experimental setups using different substrates were compared. It must be noted that this approach is not ideal, nor scientifically robust, however can still provide some insight into the effect the biocatalyst has on MFC power output.
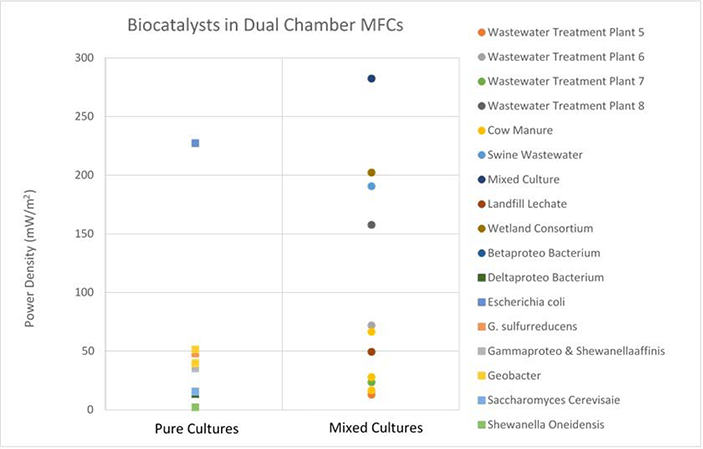
Figure 5 Dual chamber MFC experimental setups, showing maximum power density for pure and mixed microbial cultures. 25 data points are presented. The relationship between pure and mixed culture biocatalysts in dual chamber MFCs is far less profound.

Figure 6 Data summarised from Figures 4 and 5, to highlight the differences in maximum power density produced by single and dual chamber MFCs, when pure and mixed culture biocatalysts are used.
From Figure 6, the difference in power output between single and dual chamber MFCs is shown clearly. Across all substrates used, single chamber configurations consistently produce more power than dual chamber MFCs. This trend is likely attributed to the PEM membrane present in dual chamber configurations, adding significant additional internal resistance to the MFC.
Algal Substrates in MFCs
Using the data obtained from this metastudy, to ensure a valid comparison is made, the investigation of algae as a substrate in MFCs, was restricted to dual chamber setups, using mixed culture biocatalysts. From Figure 7 below, the average power density achieved from algal fuelled MFCs is approximately 70 mW/m2, while complex and simple substrates yield 30 and 280 mW/m2 respectively.

Figure 7 Results from 16 different dual chamber MFC experimental setups, all using mixed culture bacterial biocatalysts. Chlorella Vulgaris is used as the substrate in 2 setups and other substrates as shown. All simple substrates yield greater power density than algae. All complex substrates, with the exception of Swine Wastewater, yield less power density than algae.
The large difference between maximum power output for simple and complex substrates was expected and is likely attributed to the ease with which biocatalysts can metabolise simple compounds, in contrast to more complex organic structures. Algae as a substrate in MFCs does show merit, competing with wastewater substrates in terms of power output. The sustainable production of algae for use in MFCs may therefore be a viable method of electricity production.
Conclusions
This metastudy has focused particularly on the use of unconventional fuel sources, such as wastewater and algae, and the advantages of using mixed culture biocatalysts in MFCs. Through analysis of recent publications reporting experimental MFC configurations, we have identified the following trends; Analysis of dual chamber MFCs, using mixed culture biocatalysts has shown that simple substrates produce greater power output at 280 mW/m2 than algal and complex substrates, which yielded 70, and 30, mW/m2 respectively. Furthermore, we have shown that in single chamber MFC configurations, mixed culture biocatalysts yield approximately double the power output of pure strain biocatalysts. We therefore recommend the use of mixed culture microbial consortia and complex organic substrates in all future MFC applications, to maximise power output. Our research has identified that algae and wastewater show great potential as substrates in MFCs. Published data reporting the performance of such MFCs is however insufficient, we would therefore encourage the continued research in these areas.
Acknowledgments
We would like to thank the assistance of Jurgen Schulte, the students of Energy Science and Technology 2018 who peer reviewed our paper and the University of Technology Sydney. Special thanks are given to subject tutors; Blake Regan, Joshua Prichard and Marin Bell for their ongoing assistance throughout the development of this paper.
References
1. Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, et al. Microbial Fuel Cells: Methodology and Technology. Environ Sci Technol 2006;40(17):5181-5192. https://doi.org/10.1021/es0605016
2. Rahimnejad M, Adhami A, Darvari S, Zirepour A, Oh S. Microbial fuel cell as new technology for bioelectricity generation: A review. Alexandria Engineering Journal 2015;54(3):745-756. https://doi.org/10.1016/j.aej.2015.03.031
3. González del Campo A, Cañizares P, Rodrigo MA, Fernández FJ, Lobato J. Microbial fuel cell with an algae-assisted cathode: A preliminary assessment. Journal of Power Sources 2013;242:638-645. https://doi.org/10.1016/j.jpowsour.2013.05.110
4. Fernández-Marchante CM, Asensio Y, León LF, Villaseñor J, Cañizares P, Lobato J, et al. Thermally-treated algal suspensions as fuel for microbial fuel cells. Journal of Electroanalytical Chemistry 2018;814:77-82. https://doi.org/10.1016/j.jelechem.2018.02.038
5. Saratale GD, Saratale RG, Shahid MK, Zhen G, Kumar G, Shin H, et al. A comprehensive overview on electro-active biofilms, role of exo-electrogens and their microbial niches in microbial fuel cells (MFCs). Chemosphere 2017;178:534-547. https://doi.org/10.1016/j.chemosphere.2017.03.066
6. Logan BE. Exoelectrogenic bacteria that power microbial fuel cells. Nature Reviews Microbiology 2009;7:375. https://doi.org/10.1038/nrmicro2113
7. Saba B, Christy AD, Yu Z, Co AC. Sustainable power generation from bacterio-algal microbial fuel cells (MFCs): An overview. Renewable and Sustainable Energy Reviews 2017;73:75-84. https://doi.org/10.1016/j.rser.2017.01.115
8. Velasquez-Orta SB, Curtis TP, Logan BE. Energy from algae using microbial fuel cells. Biotechnol Bioeng 2009 Aug 15;103(6):1068-1076. https://doi.org/10.1002/bit.22346
9. Rabaey K, Boon N, Siciliano SD, Verhaege M, Verstraete W. Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl Environ Microbiol 2004 Sep;70(9):5373-5382. https://doi.org/10.1128/AEM.70.9.5373-5382.2004
10. Lee S, Lee K, Sorcar S, Razzaq A, Grimes C, In S. Wastewater treatment and electricity generation from a sunlight-powered single chamber microbial fuel cell. Journal of Photochemistry and Photobiology A: Chemistry. 2018;358:432-440. https://doi.org/10.1016/j.jphotochem.2017.10.030
11. Rashid N, Cui Y, Saif Ur Rehman M, Han J. Enhanced electricity generation by using algae biomass and activated sludge in microbial fuel cell. Science of The Total Environment 2013;456-457:91-94. https://doi.org/10.1016/j.scitotenv.2013.03.067
12. Yuan Y, Chen Q, Zhou S, Zhuang L, Hu P. Bioelectricity generation and microcystins removal in a blue-green algae powered microbial fuel cell. Journal of Hazardous Materials 2011;187(1):591-595. https://doi.org/10.1016/j.jhazmat.2011.01.042
13. Sonawane J, Adeloju S, Ghosh P. Landfill leachate: A promising substrate for microbial fuel cells. International Journal of Hydrogen Energy. 2017;42(37):23794-23798. https://doi.org/10.1016/j.ijhydene.2017.03.137
14. Khandelwal A, Vijay A, Dixit A, Chhabra M. Microbial fuel cell powered by lipid extracted algae: A promising system for algal lipids and power generation. Bioresource Technology 2018;247:520-527. https://doi.org/10.1016/j.biortech.2017.09.119
15. Yang N, Zhan G, Wu T, Zhang Y, Jiang Q, Li D et al. Effect of air-exposed biocathode on the performance of a Thauera –dominated mebraneless single-chamber microbial fuel cell (SCMFC). Journal of Environmental Sciences. 2018;66:216-224. https://doi.org/10.1016/j.jes.2017.05.013
16. Nguyen HTH, Kakarla R, Min B. Algae cathode microbial fuel cells for electricity generation and nutrient removal from landfill leachate wastewater. International Journal of Hydrogen Energy 2017;42(49):29433-29442. https://doi.org/10.1016/j.ijhydene.2017.10.011
17. Fischer F. Photoelectrode, photovoltaic and photosynthetic microbial fuel cells. Renewable and Sustainable Energy Reviews. 2018;90:16-27. https://doi.org/10.1016/j.rser.2018.03.053
18. Hou Q, Nie C, Pei H, Hu W, Jiang L, Yang Z. The effect of algae species on the bioelectricity and biodiesel generation through open-air cathode microbial fuel cell with kitchen waste anaerobically digested effluent as substrate. Bioresource Technology 2016;218:902-908. https://doi.org/10.1016/j.biortech.2016.07.035
19. Kakarla R, Min B. Photoautotrophic microalgae Scenedesmus obliquus attached on a cathode as oxygen producers for microbial fuel cell (MFC) operation. International Journal of Hydrogen Energy 2014;39(19):10275-10283. https://doi.org/10.1016/j.ijhydene.2014.04.158
20. Wang H, Bernarda A, Huang C, Lee D, Chang J. Micro-sized microbial fuel cell: A mini-review. Bioresource Technology. 2011;102(1):235-243. https://doi.org/10.1016/j.biortech.2010.07.007
21. Qian F, Morse D. Miniaturizing microbial fuel cells. Trends in Biotechnology. 2011;29(2):62-69. https://doi.org/10.1016/j.tibtech.2010.10.003
22. Catal T, Li K, Bermek H, Liu H. Electricity production from twelve monosaccharides using microbial fuel cells. Journal of Power Sources 2008;175(1):196-200. https://doi.org/10.1016/j.jpowsour.2007.09.083
23. Gajda I, Greenman J, Melhuish C, Ieropoulos I. Self-sustainable electricity production from algae grown in a microbial fuel cell system. Biomass and Bioenergy 2015;82:87-93. https://doi.org/10.1016/j.biombioe.2015.05.017
24. Pilatowsky I, Romero RJ, Isaza CA, Gamboa SA, Sebastian PJ, Rivera W. Thermodynamics of fuel cells. InCogeneration Fuel Cell-Sorption Air Conditioning Systems 2011 (pp. 25-36). Springer London. https://doi.org/10.1007/978-1-84996-028-1_2
25. Zielke EA. Thermodynamic analysis of a single chamber microbial fuel cell. Poster presentation, Humboldt State University, May. 2006 May 5;5.
26. Xing D, Zuo Y, Cheng S, Regan JM, Logan BE. Electricity Generation by Rhodopseudomonas palustris DX-1. Environ Sci Technol 2008;42(11):4146-4151. https://doi.org/10.1021/es800312v
27. Zheng W, Cai T, Huang M, Chen D. Comparison of electrochemical performances and microbial community structures of two photosynthetic microbial fuel cells. Journal of Bioscience and Bioengineering 2017;124(5):551-558. https://doi.org/10.1016/j.jbiosc.2017.05.013
28. Qi X, Ren Y, Liang P, Wang X. New insights in photosynthetic microbial fuel cell using anoxygenic phototrophic bacteria. Bioresource Technology. 2018;258:310-317. https://doi.org/10.1016/j.biortech.2018.03.058
29. Nimje V, Chen C, Chen C, Chen H, Tseng M, Jean J et al. Glycerol degradation in single-chamber microbial fuel cells. Bioresource Technology. 2011;102(3):2629-2634. https://doi.org/10.1016/j.biortech.2010.10.062
30. Sai Chaithanya M, Thakur S, Sonu K, Das B. Preliminary investigation of single chamber single electrode microbial fuel cell using sewage sludge as a substrate. IOP Conference Series: Materials Science and Engineering. 2017;263:032008. https://doi.org/10.1088/1757-899X/263/3/032008
31. Saratale RG, Kuppam C, Mudhoo A, Saratale GD, Periyasamy S, Zhen G, et al. Bioelectrochemical systems using microalgae – A concise research update. Chemosphere 2017;177:35-43. https://doi.org/10.1016/j.chemosphere.2017.02.132
32. Gonzalez del Campo A, Perez JF, Cañizares P, Rodrigo MA, Fernandez FJ, Lobato J. Study of a photosynthetic MFC for energy recovery from synthetic industrial fruit juice wastewater.
33. International Journal of Hydrogen Energy 2014;39(36):21828-21836. https://doi.org/10.1016/j.ijhydene.2014.07.055