PAM Review: Energy Science & Technology, Vol. 5
ISSN 2205-5231 | Published by UTS ePRESS | https://epress.lib.uts.edu.au/student-journals/index.php/PAMR/index
Evaluating Algae as an Alternative Fuel for Chemical Looping Combustion
Catherine Williams1*, Sam Bentley2, Chris Peramatukorn3, Hamza Rafi4
University of Technology Sydney, Faculty of Science, PO Box 123, Ultimo NSW 2017, Australia
1 catherine.williams@student.uts.edu.au
2 samuel.bentley-1@student.uts.edu.au
3 chris.peramatukorn@student.uts.edu.au
4 hamza.rafi@student.uts.edu.au
Corresponding author:Catherine Williams, University of Technology Sydney, Faculty of Science, PO Box 123, Ultimo NSW 2017, Australia; catherine.williams@student.uts.edu.au
DOI: http://dx.doi.org/10.5130/pamr.v5i0.1493
Citation: Williams, C., Bentley, S., Peramatukorn, C. and Rafi, H. 2018. Evaluating Algae as an Alternative Fuel for Chemical Looping Combustion. PAM Review: Energy Science & Technology , Vol. 5, pp. 37-55. http://dx.doi.org/10.5130/pamr.v5i0.1493
© 2018 by the author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License (https://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Abstract
Chemical Looping Combustion (CLC) and Chemical Looping with Oxygen Uncoupling (CLOU) are low-pollution energy generation techniques conventionally utilizing natural gas or synthetic gas as fuel. Using a redox reaction of metal oxides in dual fluidised beds, CO2 can be captured and prevented from entering the atmosphere at efficiencies up to 80% [4,8]. Algae is a sustainable source of biofuel with the additional benefit of carbon capture through photosynthesis [7]. This Meta-Study attempts to determine the viability of algae as CLC/CLOU reactor fuel for long term sustainable energy generation by identifying trends in different fuels and reactants to see if algae fuel can produce an acceptable output and identify areas of weakness. Energy balance calculations were performed as well as thermal energy output, processing energy and enthalpy values. Graciliara sp. and Chlorella Vulgaris made the most effective fuels for CLC and CLOU respectively due to the low amount of algae required to produce fuel. For CLC, 3.57kg Graciliara sp. produced 1kg fuel. For CLOU, 1.7kg Chlorella Vulgaris produced 1kg fuel. CLOU was the most mass efficient with an energy/mass efficiency ratio of 11600kJ/kg compared to CLC’s 15.7kJ/kg. The energy balance ratio analysis of the production of algal fuel also identified Chlorella Vulgaris as the best fuel, with an EBR of -0.4 in CLOU. In terms of evaluating output, CLOU’s energy/mass efficiency ratio surpassed a modern coal plant [45], whose value was 2840 kJ/kg. The defining factor was the enthalpy of reaction.
Keywords
Chemical; Looping; Combustion; CLC; Algae; Biofuel; CSS
Introduction
Concerns over global climate change and increasing pollution levels have led to increased effort into the research of renewable energy generation [13]. While most efforts have gone into the development of carbon neutral energy sources such as wind and solar power, carbon capture and carbon storage techniques for combustion-based generators are also being investigated [5,22,35,54]. Currently, three main processes are used to capture and store gaseous carbon dioxide that is generated during combustion. These include post-combustion, oxy-fuel combustion, and pre-combustion [36,37,38]. These processes, while efficient up to approximately 80% capture [4,8], (as seen in Fig. 1), require significant energy to initiate and sustain them.
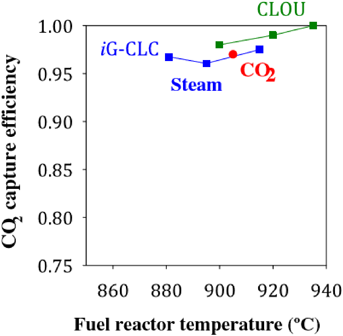
Figure 1 CO2 capture efficiency vs fuel reactor temperature [8]
CLC is a flameless, cyclic energy generation and carbon capture technique that has been considered viable for mitigating the economic, environmental and energy costs of CO2 capture and power generation [15,16,20]. The CLC process consists of two interconnected fluidized beds (layers of fluidized solid) and two main reactors [15,16,19]. An oxygen carrier, traditionally a metal oxide, reduces via combustion with a hydrocarbon fuel within the fuel reactor and is then sent to be re-oxidized in the air reactor before being sent to the fuel reactor. This cycle repeats continuously during the operation of the CLC system [17,21].
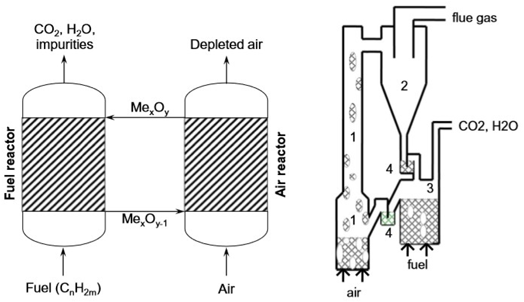
Figure 2 CLC process schematic [21]
The oxygen carrier is circulated between two fluidized beds, which eliminates the need for the fuel and air to be in contact with each other. Hence, the products of combustion (carbon dioxide and water vapor) are not mixed with the rest of the fuel gases and almost pure carbon dioxide can be obtained [8]. This reaction cycle can be described by the chemical equations below:
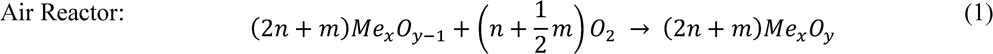
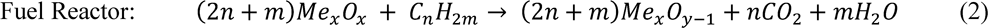
The total amount of heat released from reactions (1) and (2) can readily reach enthalpies of 30kJ/g [53] (varying upon oxygen carrier) which is comparable to more traditional combustion techniques that require contact of fuel and oxygen, such as the regular atmospheric combustion of methane which produces 50kJ/g of heat [53].
Various CLC reactor types exist, the use of which is determined by the particular fuel used. Gaseous fuels such as natural gas or synthetic gas are combusted through the common CLC process [14,16]. Solid fuels are either directly combusted by the In-situ Gasification Chemical Looping Combustion (iG-CLC) or Chemical Looping with Oxygen Uncoupling (CLOU) process, or indirectly combusted through Syngas CLC [12,18,20]. The iG-CLC process works without a gasifier and therefore, no oxygen is required.
Solid fuel is mixed with the oxygen carrier which reacts with the gasification products of the fuel. Gasifying agents are then introduced into the fuel reactor to fluidise the fuel reactor [12,19]. The CLOU process involves segregation of the oxygen carrier so that gaseous oxygen is available for the combustion of the solid fuel. Once combustion occurs, the oxygen carrier is re-oxidised in the fuel reactor [26]. Combustion of liquid fuels is a new area for CLC technology and as of this point in time has been focused upon non-sulphurous fuels such as bitumen and asphalt [24]. Current research is being conducted upon the combustion of heavy fuels and vacuum residues [20].
| Crop | Oil Yield (L/ha) | Land Area required (M ha) | Existing US cropping area (%) |
|---|---|---|---|
| Corn | 172 | 1540 | 846 |
| Soybean | 446 | 594 | 326 |
| Canola | 1190 | 223 | 122 |
| Jatropha | 1892 | 140 | 77 |
| Coconut | 2689 | 99 | 54 |
| Oil Palm | 5950 | 45 | 24 |
| Microalgaea | 136,900 | 2 | 1.1 |
| Microalgaeb | 58,700 | 4.5 | 2.5 |
In recent years, sustainable alternatives to coal and oil have been explored [2,7,10,23,54], like solar power. While solar power enables sustainable power generation with little damage to the environment there are downfalls that hinder its widespread use. Previous studies in CLC have investigated fuels such as natural gas, coal and a variety of biomass such as pine saw dust [6]. In terms of CLC, the use of algal- based fuels in particular has potential [3]. The low CO2 output of the reactor and CO2 absorption of the algae may result a power system with net negative CO2 output while maintaining a positive energy output.
As can be seen in Table 1. algae, particularly microalgae species, are promising sources of biofuels. Compared to other sources of biomass, oil yields for algae are greater per unit of land mass for fuel production [8]. However, oil yields and the compositions of the fuels produced from algae vary as there are several thousand different algae species [1,3]. Algae are eukaryotic organisms made mostly of chlorophyll which can be categorized by two main groups.
Macroalgae, also known as seaweeds which are multicellular organisms of macro-size (typically ranging from 0.5 – 60 meters in length) that live in marine environments such as seawater, wastewater [41,42]. The conversion of macroalgae to biogas can occur through various processes such as thermal treatment and fermentation, but the highest yield percentage is achieved through anaerobic digestion, which can produce up to 90% methane [42,44].
Microalgae are unicellular organisms of much smaller size (typically ranging from nanometers to millimeters in length) that can survive in extreme temperatures and with minimal water availability [41,42,43]. Macroalgae are usually considered for producing biogas which can yield up to 80% of the energy content of petroleum-based fuels, whilst microalgae species favor the production of biodiesel due to their high percentage of lipids compared to macroalgae [2,9,11,51].
The aim of this meta study is to evaluate various algae species for their use in CLC as well as to define key parameters for maximum efficient energy production.
Methods
Throughout this meta study ‘Google Scholar’, ‘UTS online library’, ‘Scopus’, ‘ProQuest Science and Technology’, ‘Web of Science Core Collection’ and ‘Elsevier Science Direct’ were used to find relevant articles. These databases were filtered using search terms ‘Chemical Looping Combustion’, ‘Algae’ and ‘Biofuels’ to identify original-research English language articles which had been published in a scientific journal. Desirable articles ideally focused on a thermodynamic aspect of the CLC Process, which could include, but was not limited to; the design and operating conditions of existing CLC systems for use in mathematical analysis, algae properties such as composition and oil yields that could affect calorific value or thermal energy calculations and thermodynamic values such as heat capacity for the calculations used in this study.
Potentially biased reports, such as those from environmental groups or private agencies, were examined and compared to unbiased technical documents from scientific research institutions. During the article and journal assessment period, all conflicts of interest between team members regarding the uncertainty of eligibility was resolved through discussion.
2.2 Data Processing
Algae types are listed, along with their fuel conversion ratios. Reactor properties such as reactor thermal energy output, fuel flow and pressure are collected. In the case of CLC, pressure is used to calculate the density of the gaseous fuel to determine the mass flow [49,50]. In the case of CLOU, since the fuel is solid, this step is not required as the mass flow is already in the correct unit.
The ratio of algae conversion into biofuel is taken from papers. This is used to determine the mass of algae required to produce 1 kg of fuel.
The outputs for the whole system over the course of 24 hrs (86400 seconds) are then simulated to determine which algae is the most effective for this reactor. This approach is taken to use time as the basis for measuring generation instead of mass used, which varies between each algae and therefore would lead to different operating times.


| Algae | Conversion Ratio (kg Algae > kg fuel) |
|---|---|
| Chlorella sorokiniana (CLOU) | 4.54 |
| Chlorella vulgaris (CLOU) | 1.7 |
| Dunaliella salina (CLOU) | 2.27 |
| Nannochloropsis oculata (CLOU) | 3.37 |
| Scenedesmus quadricauda (CLOU) | 5.43 |
| Tetraselmis suecica (CLOU) | 4.35 |
| Chaetoceros muelleri (CLOU) | 2.98 |
| Thalassiosira pseudonana (CLOU) | 4.85 |
| Ellipsoidion sp. (CLOU) | 3.65 |
| Ulva sp.1 (CLC) | 6.66 |
| Ulva sp.2 (CLC) | 12.5 |
| Ulva sp.3 (CLC) | 5.2 |
| Ulva sp.4 (CLC) | 8.33 |
| Ulva sp.5 (CLC) | 6.66 |
| Ascophyllium (CLC) | 9.09 |
| Laminara h (CLC) | 4.35 |
| Lamiara sacch (CLC) | 4.54 |
| Graciliaria sp. (CLC) | 3.57 |
| Sargassum fl. (CLC) | 5.55 |
Note that a fuel processing energy cost can be determined by multiplying the denominator of this equation to simulate the energy requirements of transporting/converting the fuel. This changes the result into “Net Energy Output (J)”

The most important equation in this study is the one to determine the mass efficiency of the reactor-i.e. how much energy you get for how much fuel you burn. Since this is a ratio, it filters out the effects of order of magnitude and allows a valid comparison.

To be effective, a reactor must at least approach the mass efficiency of commonly used plants and produce a comparable amount of power when scaled up to the same fuel input as commonly used plants. In this case, the plant used as a standard was a coal generator from the Heilbronn plant in Germany [43]. For these tests, the most effective algae for the CLC and CLOU cases were used. This is calculated to provide an easy way of showing the energy density of the fuels. While mass efficiency avoids the issue of order of magnitude, since it is a ratio, total energy output must be determined differently. The fuel flows of each reactor were adjusted to match the value of the coal generator and the energy output recalculated:

Specific Enthalpy due to algae mass was calculated from the enthalpy of reaction using standard tables and Sabatier’s reaction for methane. Solid fuel specific enthalpy couldn’t be calculated due to complexity and lack of available data:

Energy Balance Ratios were calculated based off production cost assumptions from [32], where two conditions for raceway ponds and PBRs were presented. For the raceway ponds the base case gave one watt per 10 grams of algae produced for power input and the maximum case gave one watt per twenty grams. PBRs had a base case of 500 watts per twenty grams of algae produced and the maximum case gave 50 watts per 40 grams produced. This was then factored and multiplied by our calculated mass of algae required to power a 10 kW and 1.5 kW CLC. The ratio was then calculated:

Results and Discussion
Generator fuel/type compared to mass efficiency
Though the CLC reactor types follow the same basic principles, the various ways in which different CLC fuels are treated can affect the overall energy produced per mass of the fuel. Table 4 illustrates the effect the various CLC processes would have upon the treatment of various algae fuels.
For the CLC labelled values, it is assumed that the algae has been converted to methane prior to combustion within a conventional CLC reactor. The assumption is only made for the purposes of accurately comparing the CLC reactor’s performance, as this is not a measurement of net energy- only thermal energy output per the mass of the algae. For the CLOU values it is assumed that a solid fuel biomass is used in the fuel reactor and that the main constituent for combustion is the lipid content of the material.
It appears that CLOU processing of algae biomass provides a comparable energy output to coal. However, the lipid content of the algae only amounts to 20-30% of its dry weight on average, so much of it is turned into waste products during combustion [34]. Comparatively, algae that has been converted to either gaseous or liquid fuels produces very little waste product after combustion. Though these results would indicate that the CLOU is best for mass efficiency, it is worth noting that the various treatments and production process to produce the type of algae fuel required can vary greatly.
| Generator | Thermal Energy per Kg material burnt (kJ/kg) |
|---|---|
| Heilbronn coal plant | 2840 |
| Tri-generator-LPG | 3.48 |
| Graciliaria sp.(CLC) | 15.7 |
| Ulva sp.1 (CLC) | 8.44 |
| Ulva sp.2 (CLC) | 4.49 |
| Ulva sp.3 (CLC) | 10.8 |
| Ulva sp.4 (CLC) | 6.74 |
| Ulva sp.5 (CLC) | 8.44 |
| Ascophyllium (CLC) | 6.18 |
| Laminara h (CLC) | 12.9 |
| Lamiara sacch (CLC) | 12.4 |
| Sargassum fl. (CLC) | 10.1 |
| Chlorella sorokiniana (CLOU) | 4330 |
| Chlorella vulgaris (CLOU) | 11600 |
| Dunaliella salina (CLOU) | 8650 |
| Nannochloropsis oculata (CLOU) | 5830 |
| Scenedesmus quadricauda (CLOU) | 3620 |
| Tetraselmis suecica (CLOU) | 4510 |
| Chaetoceros muelleri (CLOU) | 6590 |
| Thalassiosira pseudonana (CLOU) | 4050 |
Generator Type at Equal Fuel Flow/Energy Density
The fuel flows in each reactor are of different orders of magnitude. When they are equalized, the energy outputs correlate to the mass efficiency, suggesting the reaction method - as opposed to reactor scale – is the defining factor in the mass efficiency of the reactor. Fuel type is also not the defining factor, as from Table 3 the conversion factors for the CLC and CLOU algae used were 3.57 and 1.7 respectively. This difference of scale- roughly 2 times – would not equalize the outputs of each reactor.
The effect of higher fuel processing energies on the net output of generators
The lower the mass efficiency of the generator, the quicker the net energy becomes a negative given a range of processing energies. These results were derived from the mathematical method described in equation (7). Coal and CLOU require immense amounts of energy to be used in their processing for their outputs to become negative, whereas CLC fades early.
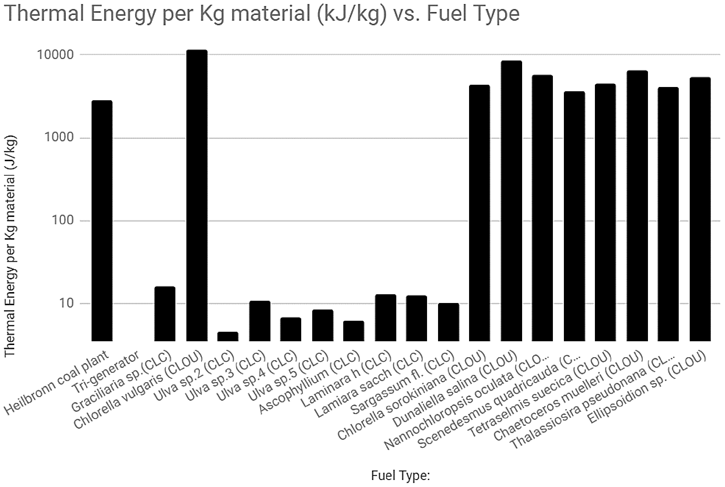
Figure 3 Thermal Energy per Kg material vs Fuel Type (including coal generator [35,43,45,46,47,48, 52]
| Reactor Type | Heilbronn | GRACE CLC | CLOU | Tri-generator |
|---|---|---|---|---|
| Fuel Flow (kg/h) | 5.66E+07 | 1.54E+04 | 2.20E-01 | 5.52E+02 |
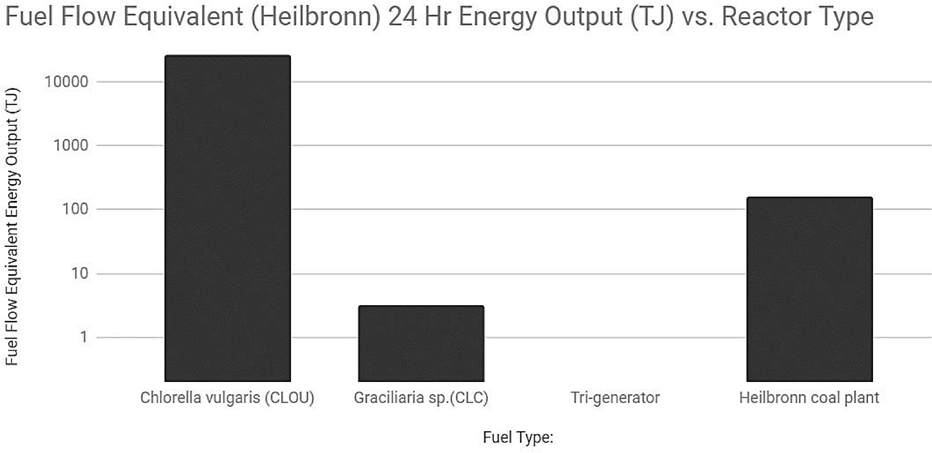
Figure 4 Fuel flow equivalent 24 Hr energy outputs for equivalent to Heilbronn fuel flow [35,43,45,46,47,48]
| Processing energy (J/kg) | CLC (Grac. sp.)ɳ𝒎 (kJ/kg) | Heilbronn Plantɳ𝒎 (kJ/kg) | CLOU (Chlor. Vul)ɳ𝒎 (kJ/kg) |
|---|---|---|---|
| 1 | 15.7 | 2840 | 11600 |
| 5 | 3.15 | 568 | 2310 |
| 10 | 1.57 | 284 | 1160 |
| 50 | 0.315 | 56.8 | 231 |
| 100 | 0.157 | 28.4 | 116 |
| 500 | 0.032 | 5.68 | 23.1 |
| 1000 | 0.016 | 2.84 | 11.6 |
| 5000 | 0.003 | 0.568 | 2.31 |
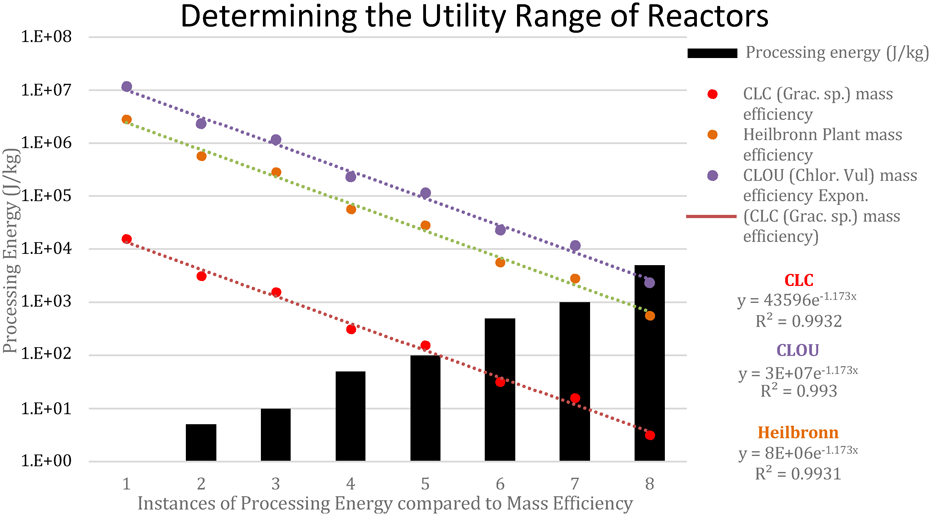
Figure 5 Fuel flow equivalent 24 Hr energy outputs for equivalent to Heilbronn fuel flow [35,43,45,46,47,48]
Enthalpy/Calorific Value/Algae Properties
Enthalpy determines the amount of heat that is released upon combustion of a fuel. Of the algae species studied above Graciliaria has the highest specific enthalpy for algae species converted to methane. In the below figure, Graciliaria sp. has a higher carbohydrate and protein composition than the Ulva species which would indicate that the higher these values are, the greater the specific enthalpy of the fuel.
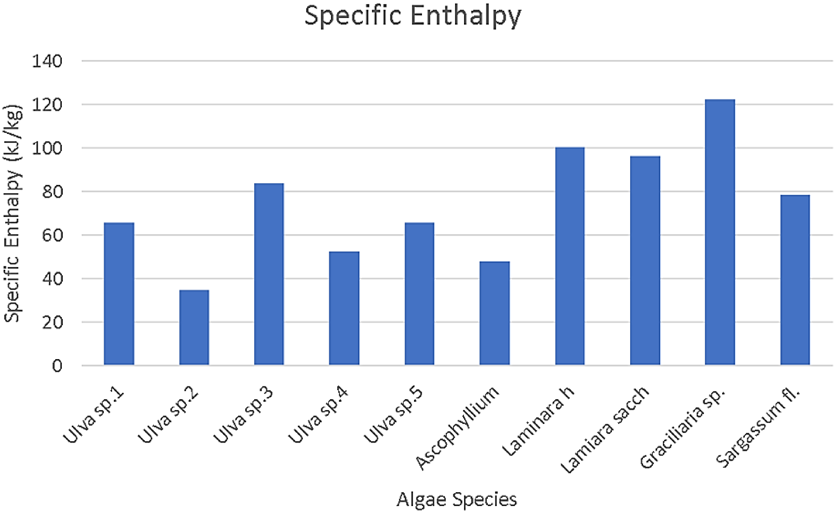
Figure 6 Specific enthalpy by algae species [1, 2, 3, 7, 10, 11]
Enthalpy determines the amount of heat that is released upon combustion of a fuel. Of the algae species studied above Graciliaria has the highest specific enthalpy for algae species converted to methane. In the below figure, Graciliaria sp. has a higher carbohydrate and protein composition than the Ulva species which would indicate that the higher these values are, the greater the specific enthalpy of the fuel.
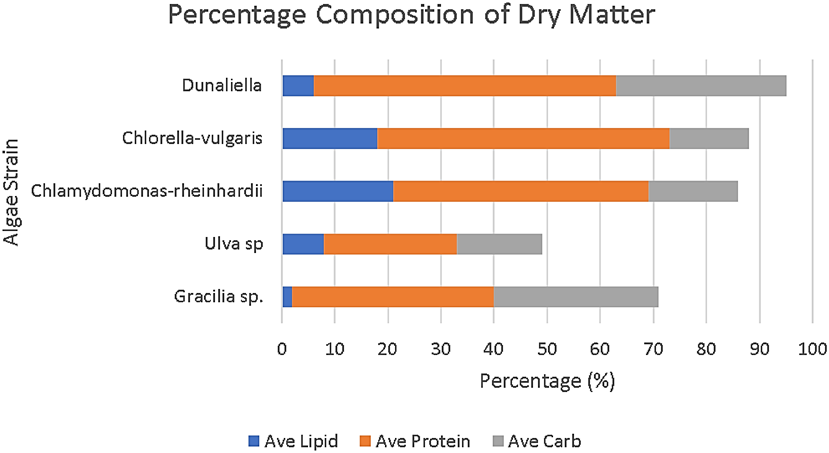
Figure 7 Chemical composition of microalgal species from [32, 33, 37, 47,48, 52]. Results are expressed as percentage of dry wt. Biomass
Algae species vary by their lipid, carbohydrate and protein compositions. Current studies show that high lipid and carbohydrate concentration contributes to calorific value, the amount of polysaccharides produced per species can be converted into bioethanol and available triacyl glycerides can be converted to biodiesel [27].
As in 3.3, the calorific value of algae compared to coal is quite low and there is much variation between algae species as well as the production processes used to cultivate algae. Current research into algae as a source for biofuel is concentrated around increasing this calorific value.
Chia et al, found that varying the concentration of phosphorus found in the Chlorella vulgaris strain increased its calorific value from 13.48 kJ/g up to 33.07 kJ/g [27]. R Slade et al, compared the various algae cultivation techniques and found that by comparing the Net energy ratio (as a measure of the energy needed to produce algae and the energy content of the algae) of algae grown through different processes, they were able to identify that Raceway Ponds are the best setups for a positive energy balance over PBR systems [32].
Energy to cultivate algae vs. Energy Output
For the following graphs and tables the generator output was taken from the 10 kW CLC and 1.5 kW CLOU models. The mass was divided by Biomass productivity [32] to find the power required to make the 10 kW energy for the day. Here, the Energy Balance Ratio is defined as the power required to produce n amount of algae for one day over the power output. This is calculated for Raceway ponds and Photo Bio Reactor systems.
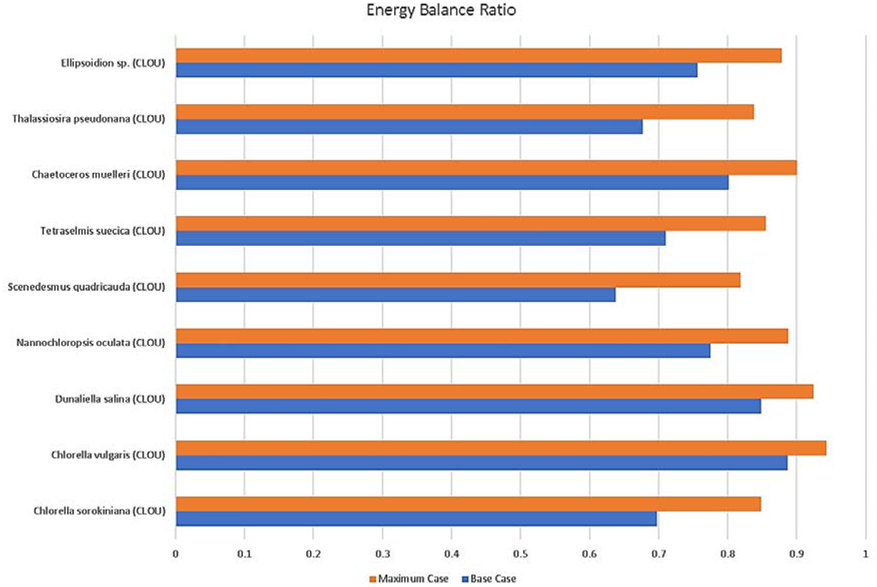
Figure 8 Energy balance ratio with CLOU reactor [32]. The blue lines indicate the use of a standard Raceway Pond setup. The orange lines indicate the use of an improved Raceway Pond [32]
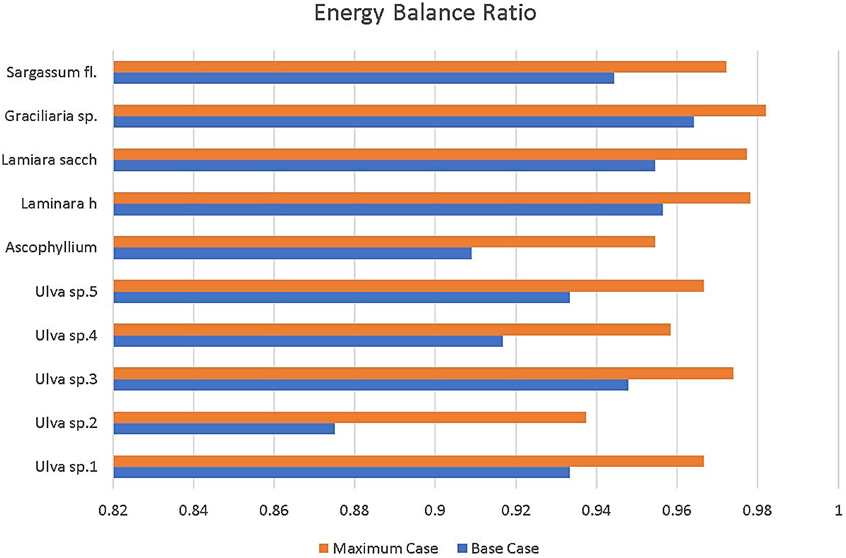
Figure 9 Energy balance ratio in CLC reactor [32]. The blue lines indicate the use of a standard Raceway Pond setup. The orange lines indicate the use of an improved Raceway Pond [32]
Raceway Ponds appear to be an ideal production process of algae for CLC. For the 1.5 kW CLOU model, the energy balance ratio calculated for power input over power output ranged from 0.64 for Scenedesmus quadricauda to 0.89 for Chlorella Vulgaris. This range was based on common raceway pond designs. For most efficient Raceway Pond design [32], the energy balance ratio ranged from 0.82 to 0.94 for the same species.
For the 10 kW CLC model, the energy balance ratio calculated for power input over power output ranged from 0.88 for Ulva sp.2 to 0.86 for Graciliaria sp. in the base case. For the most efficient raceway pond design studied in [32], the energy balance ratio ranged from 0.94 to 0.98 for the same species.
The Photobioreactor (PBR) results, in comparison, were quite poor. For the 1.5 kW CLOU model, the energy balance ratio for PBR ranged from -79.8 for Thalassiosira pseudonana to -27.3 for Chlorella Vulgaris. For the most efficient PBR design, the energy balance ratio ranged from -0.4 to -3.0 for the same species. This presents a drastic improvement as a result of better PBR system designs. However, the negative values still indicate that more energy is input than output and this is not ideal.
The same trend was observed in the 10kW CLC model, with a marked increase in improvement from the basic PBR design to an improved one. The energy balance ratio ranged from -30.3 for Ulva sp 2. to -7.9 for Graciliaria sp. in the base case. For the most efficient PBR design, the energy balance ratio ranged from -0.6 to 0.6 for the same species. Though improvements in PBR design greatly improve the EBR, the Raceway Pond method of cultivation for algae have the best EBR values overall. The best algae source from those studied is Chlorella Vulgaris.
| Algae | EBR (Base) | EBR (Maximum) |
|---|---|---|
| Chlorella sorokiniana | -74.7 | -2.8 |
| Chlorella vulgaris | -27.33 | -0.4 |
| Dunaliella salina | -36.8 | -0.9 |
| Nannochloropsis oculata | -55.2 | -1.8 |
| Scenedesmus quadricauda | -89.5 | -3.5 |
| Tetraselmis suecica | -71.5 | -2.6 |
| Chaetoceros muelleri | -48.7 | -1.5 |
| Thalassiosira pseudonana | -79.8 | -3.0 |
| Ellipsoidion sp. | -59.8 | -2.0 |
| Algae | EBR (Base) | EBR (Maximum) |
|---|---|---|
| Ulva sp.1 | -15.7 | 0.2 |
| Ulva sp.2 | -30.3 | -0.6 |
| Ulva sp.3 | -12.0 | 0.4 |
| Ulva sp.4 | -19.8 | 0 |
| Ulva sp.5 | -15.7 | 0.2 |
| Ascophyllium | -21.7 | -0.1 |
| Laminara h | -9.9 | 0,5 |
| Lamiara sacch | -10.4 | 0.4 |
| Graciliaria sp. | -7.9 | 0.6 |
| Sargassum fl. | -12.9 | 0.3 |
Conclusions
A mathematical model was produced to calculate the net output energy of biofuels in a CLC reactor produced from a range of algae. It was found that the most suitable candidate for CLC was Graciliaria sp from the amount of methane able to be converted from the biomass, with 3.57kg algae being used to form 1kg methane. Likewise, for CLOU the most suitable candidate was Chlorella Vulgaris for the same reason, with a conversion factor of 1.7kg->1kg.
The use of improved raceway ponds to breed algae could improve the energy balance ratio; resulting in an increase of efficiency, with EBR increases of 0.18-0.05 in CLOU reactors and 0.06-0.02 in CLC reactors.
Even though in CLOU much of the algae is discarded before combustion, potentially requiring a lot of energy and wasting a lot of mass, the outputs pay off due to its enthalpy of combustion. It has a mass efficiency of 11600kJ/kg, much more effective than the coal plant, with a value of 2840kJ/kg. When the energy required to process the algae rises, CLOU’s net energy only drops to negative at 5kJ/kg fuel / 2.31kJ/kg mass efficiency- at the same level, the coal generator’s energy is 5kJ/kg fuel / 0.568 kJ/kg mass efficiency. CLC drops off at 0.5kJ/kg fuel / 0.032kJ/kg mass efficiency. Even over a wide variety of fuels, each reaction type stayed within the same order of magnitude- there was not a single instance where a fuel variance raised the abilities of one reactor over another. The defining factor for a successful reactor is therefore enthalpy of combustion over all other factors including both fuel type and reactor size/fuel flow, and an algae-powered CLOU excels at it.
Acknowledgments
We would like to extend gratitude to Jurgen Schulte, Blake Regan, Martin Bell and especially Joshua Pritchard for their mentoring and critique. We would also like to thank the students in the class who participated in peer reviews throughout the creation of this paper and to UTS for its support.
References
1. Guiry M. How many species of algae are there? Journal of Phycology. 2012;48(5):1057-1063. https://doi.org/10.1111/j.1529-8817.2012.01222.x
2. Spolaore P, Joannis-Cassan C, Duran E, Isambert A. Commercial applications of microalgae. Journal of Bioscience and Bioengineering. 2006;101(2):87-96. https://doi.org/10.1263/jbb.101.87
3. Metting F. Biodiversity and application of microalgae. Journal of Industrial Microbiology & Biotechnology. 1996;17(5-6):477-489. https://doi.org/10.1007/BF01574779
4. Kvamsdal H, Jordal K, Bolland O. A quantitative comparison of gas turbine cycles with CO2 capture. Energy. 2007;32(1):10-24. https://doi.org/10.1016/j.energy.2006.02.006
5. Thomas D, Benson S. Carbon dioxide capture for storage in deep geologic formations. Amsterdam: Elsevier; 2005. https://doi.org/10.1016/b978-008044570-0/50125-2
6. Mendiara T, Abad A, de Diego L, García-Labiano F, Gayán P, Adánez J. Biomass combustion in a CLC system using an iron ore as an oxygen carrier. International Journal of Greenhouse Gas Control. 2013;19:322-330. https://doi.org/10.1016/j.ijggc.2013.09.012
7. Menetrez M. An Overview of Algae Biofuel Production and Potential Environmental Impact. Environmental Science & Technology. 2012;46(13):7073-7085. https://doi.org/10.1021/es300917r
8. Mattisson T, Keller M, Linderholm C, Moldenhauer P, Rydén M, Leion H et al. Chemical-looping technologies using circulating fluidized bed systems: Status of development. Fuel Processing Technology. 2018;172:1-12. https://doi.org/10.1016/j.fuproc.2017.11.016
9. John R, Anisha G, Nampoothiri K, Pandey A. Micro and macroalgal biomass: A renewable source for bioethanol. Bioresource Technology. 2011;102(1):186-193. https://doi.org/10.1016/j.biortech.2010.06.139
10. Jacob A, Xia A, Murphy J. A perspective on gaseous biofuel production from micro-algae generated from CO 2 from a coal-fired power plant. Applied Energy. 2015;148:396-402. https://doi.org/10.1016/j.apenergy.2015.03.077
11. Chen H, Zhou D, Luo G, Zhang S, Chen J. Macroalgae for biofuels production: Progress and perspectives. Renewable and Sustainable Energy Reviews. 2015;47:427-437. https://doi.org/10.1016/j.rser.2015.03.086
12. Adánez J, Abad A, Mendiara T, Gayán P, de Diego L, García-Labiano F. Chemical looping combustion of solid fuels. Progress in Energy and Combustion Science. 2018;65:6-66. https://doi.org/10.1016/j.pecs.2017.07.005
13. Kaarstad O. Emission-free fossil energy from Norway. Energy Conversion and Management. 1992;33(5-8):781-786. https://doi.org/10.1016/0196-8904(92)90084-A
14. Mattisson T, Lyngfelt A, Cho P. The use of iron oxide as an oxygen carrier in chemical-looping combustion of methane with inherent separation of CO2. Fuel. 2001;80(13):1953-1962. https://doi.org/10.1016/S0016-2361(01)00051-5
15. Jerndal E, Mattisson T, Lyngfelt A. Thermal Analysis of Chemical-Looping Combustion. Chemical Engineering Research and Design. 2006;84(9):795-806. https://doi.org/10.1205/cherd05020
16. Rydén M, Moldenhauer P, Mattisson T, Lyngfelt A, Younes M, Niass T et al. Chemical-Looping Combustion with Liquid Fuels. Energy Procedia. 2013;37:654-661. https://doi.org/10.1016/j.egypro.2013.05.153
17. Adánez J, Gayán P, Adánez-Rubio I, Cuadrat A, Mendiara T, Abad A et al. Use of Chemical- Looping processes for coal combustion with CO2 capture. Energy Procedia. 2013;37:540-549. https://doi.org/10.1016/j.egypro.2013.05.140
18. Abad A, Mendiara T, Gayán P, García-Labiano F, de Diego L, Bueno J et al. Comparative Evaluation of the Performance of Coal Combustion in 0.5 and 50 kW th Chemical Looping Combustion Units with Ilmenite, Redmud or Iron Ore as Oxygen Carrier. Energy Procedia. 2017;114:285-301. https://doi.org/10.1016/j.egypro.2017.03.1170
19. Adanez J, Abad A, Garcia-Labiano F, Gayan P, de Diego L. Progress in Chemical-Looping Combustion and Reforming technologies. Progress in Energy and Combustion Science. 2012;38(2):215-282. https://doi.org/10.1016/j.pecs.2011.09.001
20. Nandy A, Loha C, Gu S, Sarkar P, Karmakar M, Chatterjee P. Present status and overview of Chemical Looping Combustion technology. Renewable and Sustainable Energy Reviews. 2016;59:597-619. https://doi.org/10.1016/j.rser.2016.01.003
21. Adánez J, Gayán P, Celaya J, de Diego L, García-Labiano F, Abad A. Chemical Looping Combustion in a 10 kWthPrototype Using a CuO/Al2O3Oxygen Carrier: Effect of Operating Conditions on Methane Combustion. Industrial & Engineering Chemistry Research. 2006;45(17):6075-6080. https://doi.org/10.1021/ie060364l
22. Hossain MM, de Lasa HI. Chemical-looping combustion (CLC) for inherent CO2 separations—a review. Chemical Engineering Science 2008;63(18):4433-4451. https://doi.org/10.1016/j.ces.2008.05.028
23. Gao, D., Li, Z., Liu, P., Zhao, J., Zhang, Y. and Li, C. (2018). A coordinated energy security model taking strategic petroleum reserve and alternative fuels into consideration. Energy, 145, pp.171- 181. https://doi.org/10.1016/j.energy.2017.11.097
24. Cao Y, Lia B, Zhao HY, Lin CW, Sit SP, Pan WP. Investigation of asphalt (Bitumen) fueled chemical looping combustion using durable copper-based oxygen carrier. Energy Procedia 2011;4:457–64. https://doi.org/10.1016/j.egypro.2011.01.075
25. Rydén M, Lyngfelt A, Mattisson T. CaMn0.875Ti0.125O3 as oxygen carrier for ` with oxygen uncoupling (CLOU)—Experiments in a continuously operating fluidized-bed reactor system. International Journal of Greenhouse Gas Control 2011;5(2):356-366. https://doi.org/10.1016/j.ijggc.2010.08.004.
26. Chia MA, Lombardi AT, Melão G, da Graça M. Calorific values of Chlorella vulgaris (Trebouxiophyceae) as a function of different phosphorus concentrations. Phycological Research. 2013 Oct 1;61(4):286-91. https://doi.org/10.1111/pre.12026
27. M. Alvarado-Morales, A. Boldrin, D. B. Karakashev, S. L. Holdt, I. Angelidaki, and T. Astrup, “Life cycle assessment of biofuel production from brown seaweed in Nordic conditions,” Bioresource technology, vol. 129, pp. 92-99, 2013. https://doi.org/10.1016/j.biortech.2012.11.029
28. Darzins, P. Pienkos, and L. Edye, “Current status and potential for algal biofuels production,” A report to IEA Bioenergy Task, vol. 39, 2010.
29. O. Alabi, “Microalgae technologies & processes for biofuels/bioenergy production in British Columbia: Current Technologies, Suitability & Barriers to Implementation,” January 14, 2009 2009.
30. L. Zhu, E. Hiltunen, E. Antila, J. Zhong, Z. Yuan, and Z. Wang, “Microalgal biofuels: Flexible bioenergies for sustainable development,” Renewable and Sustainable Energy Reviews, vol. 30, pp. 1035-1046, 2014. https://doi.org/10.1016/j.rser.2013.11.003
31. P. Biller, B. K. Sharma, B. Kunwar, and A. B. Ross, “Hydroprocessing of bio-crude from continuous hydrothermal liquefaction of microalgae,” Fuel, vol. 159, pp. 197-205, 2015. https://doi.org/10.1016/j.fuel.2015.06.077
32. Slade R, Bauen A. Micro-algae cultivation for biofuels: cost, energy balance, environmental impacts and future prospects. Biomass and bioenergy. 2013 Jun 1;53:29-38. https://doi.org/10.1016/j.biombioe.2012.12.019
33. Boundy B, Diegel SW, Wright L, Davis SC. Biomass Energy Data Book, US Dep. Of Energy, 2011. ORNL/TM-2011/446, http://cta. ornl. gov/bedb/download. shtml. https://doi.org/10.2172/1050890
34. Rocca S, Agostini A, Giuntoli J, Marelli L. Biofuels from algae: technology options, energy balance and GHG emissions. JRC Available at: http://publications. jrc. ec. europa. eu/repository/bitstream/JRC98760/algae_biofuels_report_21122015. pdf. 2015.
35. Adánez-Rubio I, Abad A, Gayán P, de Diego LF, García-Labiano F, Adánez J. Biomass combustion with CO2 capture by chemical looping with oxygen uncoupling (CLOU). Fuel Process Technol 2014;124:104-114. https://doi.org/10.1016/j.fuproc.2014.02.019
36. Stanger R, Wall T, Spörl R, Paneru M, Grathwohl S, Weidmann M, et al. Oxyfuel combustion for CO2 capture in power plants. International Journal of Greenhouse Gas Control; Special Issue commemorating the 10th year anniversary of the publication of the Intergovernmental Panel on Climate Change Special Report on CO2 Capture and Storage 2015;40:55-125. https://doi.org/10.1016/j.ijggc.2015.06.010
37. Wang Y, Zhao L, Otto A, Robinius M, Stolten D. A Review of Post-combustion CO2 Capture Technologies from Coal-fired Power Plants. Energy Procedia; 13th International Conference on Greenhouse Gas Control Technologies, GHGT-13, 14-18 November 2016, Lausanne, Switzerland 2017;114:650-665. https://doi.org/10.1016/j.egypro.2017.03.1209
38. Jansen D, Gazzani M, Manzolini G, Dijk Ev, Carbo M. Pre-combustion CO2 capture. International Journal of Greenhouse Gas Control; Special Issue commemorating the 10th year anniversary of the publication of the Intergovernmental Panel on Climate Change Special Report on CO2 Capture and Storage 2015;40:167-187. https://doi.org/10.1016/j.ijggc.2015.05.028
39. T. Burton, H. Lyons, Y. Lerat, M. Stanley, and M. B. Rasmussen, “A review of the potential of marine algae as a source of biofuel in Ireland,” Dublin: Sustainable Energy Ireland-SEI2009.
40. U.S.DOE, “National Algal Biofuels Technology Roadmap. U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Biomass Program.,” Workshop and Roadmap sponsored by the U.S. Department of Energy Office of Energy Efficiency and Renewable Energy Office of the Biomass Program 2010.
41. S.-K. Kim, “Handbook of Marine Macroalgae: Biotechnology and Applied Phycology,” John Wiley & Sons, 04/nov/2011. https://doi.org/10.1002/9781119977087
42. Hughes, et. al. “Biogas from Macroalgae: is it time to revisit the idea?”, Biotechnology for Biofuels (online), 2012. https://doi.org/10.1186/1754-6834-5-86
43. Starkloff, R., Alobaid, F., Karner, K., Epple, B., Schmitz, M. and Boehm, F. (2015). Development and validation of a dynamic simulation model for a large coal-fired power plant. Applied Thermal Engineering, 91, pp.496-506. https://doi.org/10.1016/j.applthermaleng.2015.08.015
44. Salerno M., et. al., “Biogas Production from Algae Biomass Harvested at Wastewater Treatment Ponds”, 2009 Bioenergy Engineering Conference Sponsored by American Society of Agricultural and Biological Engineers 2009. https://doi.org/10.13031/2013.28877
45. Ge, Y., Tassou, S., Chaer, I. and Suguartha, N. (2009). Performance evaluation of a tri-generation system with simulation and experiment. Applied Energy, 86(11), pp.2317-2326. https://doi.org/10.1016/j.apenergy.2009.03.018
46. Lyngfelt, A., Kronberger, B., Adanez, J., Morin, J. and Hurst, P. (2005). The grace projectDevelopment of oxygen carrier particles for chemical-looping combustion. Design and operation of a 10 kW chemical-looping combustor. Greenhouse Gas Control Technologies 7, pp.115-123. https://doi.org/10.1016/B978-008044704-9/50013-6
47. Huntley M, Redalje D. CO2 Mitigation and Renewable Oil from Photosynthetic Microbes: A New Appraisal. Mitigation And Adaptation Strategies For Global Change 2006;12:573-608. https://doi.org/10.1007/s11027-006-7304-1
48. Alamsjah M, Abdillah A, Mustikawati H, Atari S. Screening of biodiesel production from waste tuna oil (Thunnus sp.), seaweed Kappaphycus alvarezii and Gracilaria sp. 2017. https://doi.org/10.1063/1.5004286
49. Natural gas — Calculation of compression factor — Part 2: Calculation using molar-composition analysis. Isoorg 2018.
50. Starling K, Savidge J. Compressibility factors of natural gas and other related hydrocarbon gases. Arlington, Va: AGA, American Gas Association; 1994.
51. Milano J., et. al. “Microalgae biofuels as an alternative to fossil fuel for power generation”, Renewable and Sustainable Energy Reviews 2016;58:180-197. https://doi.org/10.1016/j.rser.2015.12.150
52. Vanegas C, Bartlett J. Green energy from marine algae: biogas production and composition from the anaerobic digestion of Irish seaweed species. Environmental Technology 2013;34:2277-2283. https://doi.org/10.1080/09593330.2013.765922
53. Bhoje R., Kale G. R, Labhsetwar “Chemical looping combustion of methane: A technology development view” CSIR National Environmental Engineering Research Institute India 2008 https://doi.org/10.1155/2013/949408
54. Osueke C. O., Onyekachi E. M. “Combating Problems with Solar Power: A cost Effective Improvement on the Conversion Efficiency of Solar Panels” International Journal of Scientific and Engineering Research 2011;2:10

