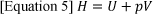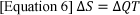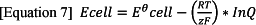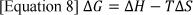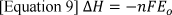Thermodynamic Effects of Nanotechnological Augmentation of Hydrogen Fuel Cells
Dennis Tran 1, Geoffrey Tanner 2, Kane Yang 3 and Reece Woodley 4*
University of Technology Sydney, P.O Box 123, MaPS, Broadway NSW 2007.
1 E-Mail: Dennis.tran@student.uts.edu.au
2 E-Mail: Geoffrey.B.Tanner@student.uts.edu.au
3 E-Mail: Kane.Yang@student.uts.edu.au
4 E-Mail: Reece.C.Woodley@student.uts.edu
* Author to whom correspondence should be addressed; E-Mail: Reece.C.Woodley@student.uts.edu
DOI: http://dx.doi.org/10.5130/pamr.v4i0.1443
Abstract: This meta-study focuses on the research regarding the use of nanotechnology in traditional fuel cells in order to increase thermodynamic efficiency through the exploitation of various thermodynamic systems and theories. The use of nanofilters and nano-structured catalysts improve the fuel cell system through the means of filtering molecules from protons and electrons significantly increases the possible output of the fuel cell and the use of nano-platinum catalysts to lower the activation energy of the fuel cell chemical reaction a notable amount resulting in a more efficient system and smaller entropy in comparison to the use of macro sized catalysts.
Keywords: Fuel Cell, Nanotechnology, Nanofilter, Hydrogen Fuel Cell, Carnot Cycle.

Copyright 2017 by the authors. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 Unported (CC BY 4.0) License (https://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
1. Introduction
In the modern world, alternative energy sources are on the rise and there is a large variety of methods been developed, solar and wind been two well-known examples. A source of energy been investigated is fuel cells, which use chemical reactions to produce a voltage and includes many variations, like the Proton Exchange Membrane (PEM) fuel cell1.
1.1 Fuel Cells
Fuel cells are systems which convert the chemical energy of its fuel to electricity from the reaction of positively charged hydrogen ions with oxygen. It does this by catalytically breaking apart the hydrogen at the anode. The electrons flow along a load circuit towards the cathode, generating a current and then recombine with the hydrogen at the cathode. The Hydrogen ions (Protons) are drawn through the membrane of the system toward the cathode, which with the Oxygen molecules, produce water as a waste product.

Figure 1. Proton exchange membrane fuel cell (PEM) also known as polymer electrolyte membrane fuel cells. Most commonly used in portable and stationary solutions due to its lower operating temperature and pressure ranges. A leading candidate to replace aging fuel cell technology. (1) Hydrogen and Oxygen pumped into the system. (2) Excess Hydrogen is recycled back into the system. (3) Hydrogen is split at the Anode. (4) Electrons (e-) cycle around the membrane, producing a current and powering an //external circuit, while the Protons (H+, hydrogen ions) cross over the membrane. (5) The oxygen, hydrogen and electrons combine at the cathode. (6) Water is expelled from the system.
1.2 Nano Filters/Membranes
Nanofilters are specially designed membranes to filter out components at the molecular level. In a system such as PEM Fuel Cell, the membrane functions as a gate, allowing protons to pass through but nothing else, as membrane needs to allow the crossover of the hydrogen protons without allowing the electrons through as this may cause a short circuit in the system.
Nanofilters must not allow either the hydrogen gas or the oxygen gas to pass to the opposing side they are injected into the system from and it must not react with either the reducing or oxidizing environments around the cathode and anode respectively 2,3. Membranes commercially used today are Nafion, a sulfonated tetrafluoroethylene based fluoropolymer-copolymer produced in the 1960’s by Walther Grot 4. The drawback to this membrane is it is permeable to gas exchange and can’t operate a very high temperature, as they rely on liquid water to facilitate the movement of protons. Similar membranes (such Flemion or Aciplex) suffer the same drawbacks. Other “newer” polymer membranes do not have this drawback, such as using a Phosphoric Acid based membrane. These membranes can operate at temperatures of 220oC, these types of membranes are not common due to acid leeching through the system and the difficulty of mixing and creating the polymers 5.
Recently developments in the materials used to construct the membrane increase the degree to which this unwanted gas exchange occurs, such as Graphene which only allows the protons to pass through 6.
1.3 Chemistry
Fuel cells make use of moving electrons from chemical reactions, with a catalyst used to reduce the activation energy of the reaction. Hydrogen fuel cells release electrons from the anode to the cathode to create water7, shown by the equations below:

Figure 2. Half equations 1, 2, 3, 4 for the oxidation and reduction of the fuel cell during discharge7
The reactants are hydrogen gas and oxygen gas, two readily available elements in the natural environment, which is easily renewable due to its abundance on earth 8. The final product created is water, a safe chemical which can be disposed safely or recycled as a coolant, making the hydrogen fuel cells a renewable fuel cell with environmentally disposable waste.
1.4 Thermodynamics
Any system producing energy obeys the laws of thermodynamics 9. The three laws of thermodynamics govern the energy exchange that takes place in a fuel cell. Where Enthalpy and Entropy are significant in particular to fuel cells. Enthalpy is the thermodynamic quantity that states the total heat content of the system, which is the sum of all internal process in a closed system 9. For homogeneous systems, enthalpy is solely based on the size of the system as it is an extensive property 10. This is shown in equation [5], where enthalpy (H) is the sum of the internal energy of the system (U) and the product of pressure (P) and volume (V) of the system 9. The change in enthalpy in a system is equal to the heat gained or lost in the system 11. The enthalpy change (ΔH) for a reaction is a fuel cell indicates the full amount of heat released by the reaction at a constant pressure. The potential in accordance to ΔH is referred to as the thermos-neutral potential, E₀.
Entropy is the measure of molecular disorder and randomness, representing the energy within the system which is unavailable to do work for the system, also known as the second law of thermodynamics 11. The total entropy of a system increases over time, as the molecular disorder increases. Therefore, if the system is in equilibrium, the change between the initial state to the final state, the system is going through a reversible change. Since entropy represents the unavailable energy used in the system, a system of zero entropy optimises the work output of the system12. The equation for the change of entropy (ΔS) is equal to the change in enthalpy (∆Q) divided by the temperature (T) of the system11.
Exergy is defined as the maximum amount of work obtained from the system or the flow of matter when the chemical reaction in the system is brought to a reversible equilibrium, as fuel cells are not energy converters, but rather work potential generators 12. It is often also used to measure the loss of the system and is proportional to the entropy creation in the system. It can give a more accurate measure of loss compared to entropy, especially in the situation where power optimisation is the goal. In relation to thermodynamics, the max work produced from the reaction is in terms of the free-energy change of the reaction. (Table. 1)
Chemical reactions that are carried out at a constant temperature and pressure are more practical, than that are carried out at a constant temperature and volume. Hydrogen reaction that takes place in equation [3] is more thermodynamically efficient as it produces favourable free-energy, here the products of this is less than that of the reactants. The electrochemical reaction that takes place in a fuel cell determines the performance of the fuel cell, and if it is ideal. The minimum temperature for optimum operation varies, where H2 is used in low to medium temperature fuel cells, as they are limited by the noble metal electrocatalysts, as this affects the reaction rates at the anode and cathode 13.
The ideal performance of a fuel cell is determined by the Nernst equation, which represents the relationship between the ideal potential of the reaction and the ideal equilibrium potential. The Nernst equation, related to the reduction potential of an electrochemical reaction to the standard electrode potential and temperature. It is derived from changes in the Gibbs free energy, and its association with electrochemical transformation. The total cell potential equation is used to find the electric potential of a cell membrane with respect to one type of ion:
As stated, the Nernst equation is based around the total cell potential, at a defined by temperature and is given as Ecell. This is then equal sum of Eᶱcell, which is the standard cell potential, or the potential of the chemical reaction that takes place in the cell, which varies depending on the entropy in the reaction 10. Once the ideal potential of the system has been determined for standard conditions the ideal voltage can then be determined for other temperature and pressure through the use of the Nernst equation. For hydrogen oxidation, the ideal cell potential at given temperatures can be improved or increased by operating the cell at higher pressures.
The Nernst equation gives the ability to calculate when a system goes through a refined reaction its potential is increased at the entropy is minimised. For a perfect system, there needs to be zero entropy 12. The use of nanotechnology, particularly porous graphene as a filter, assists in refining the reaction and therefore increasing the potential of the system 10.
2. Method
Focus of the study included reading papers on nanomembranes and nanofilters and their application in fuel cells, other papers that analysed key parts such fuel cell entropy and nanomaterials in energy were also collected in addition to textbook references for standard equations. The tools used to acquire the reports were Scopus and Google Scholar, searches were restricted to papers and journals published since 1995 as papers published during this period was during the height of fuel cell interest and commercial notoriety 14. The scope was restricted to the Asian and North American regions as this most area produces the most relevant data. The papers chosen were experimentally based rather than other meta-study data, as these papers had too much unrelated information of the economic and social fields. Keywords used in the investigation were: Nanofilter, Nanomembrane, Nanocatalyst, Fuel Cell, Hydrogen Fuel, Platinum Catalyst, Palladium Catalyst, PEM Cell, Carnot Cycle, Electrolysis, Cathode, Anode.
During the study, experimental data was taken from their corresponding papers for original calculations to show the effects the membranes had on hydrogen fuel cells, compared to other models using current filtration methods. Calculations of surface area and volumes were based on a theoretical model of the structure.
3. Results and Discussion
Reducing the randomness of the fuel cell reduces the entropy of the system, stated by the second law of thermodynamics, therefore Nanofilters reduce the entropy of the system by controlling the rate of reaction. Porous graphene 15 is produced as a single sheet of carbon, bonded in a planar, hexagonal structure, with several carbon atoms removed to create a “pore”, shown in figure [3]16,17 which can be used as a nanofilter 18. Due to graphene’s open structure, electrons and protons can freely pass through the lattice with minimal resistance, while restricting the flow of larger molecules, which may only pass through the pores6.

Figure 3. A graphene sheet has a carbon ring removed to allow permeation of H2 molecules while filtering out any impurities or by-products larger than the pore created. This allows the ‘filtering’ of the molecules in the fuel cell system for purer and more consistent reactions.
By restricting the reactants, the oxygen and hydrogen gases react with a high precision ratio. This is due to the porous graphene membrane in the fuel cell been less prone to gas exchange 19, as well as controlling the rate of reaction due to the restrictive flow of the reactants, as demonstrated by figure [4]20. Precise control of the chemical reaction and reducing gas exchange increases the number of reactions that occur, giving cleaner reactions and overall output of the cell.

Figure 4. The efficiency of porous graphene on filtering different molecules from hydrogen gas. As the molecule size increases, the porous graphene is more successful in filtering the molecule out, under the assumption that all pores created are the same size. 6
Current from the anode to cathode, shown in figure [2] and by equations [1] and [2], is reduced by the resistance of the current carrier, due to structural interference, shown in figure [5] 21. Graphene can overcome this problem in the form of carbon nanotubes (CNTs). CNT’s structure has minimal electrical resistance due to the interior opening created by the tube, making it an ideal way to transport electrons through the electrode 22. Reducing the electrical resistance reduces electron collision with the electrode’s structure, as every electron collision causes the electrons to lose momentum and energy. By reducing electron collisions, the internal energy required to run the fuel cell is reduced, making CNT an efficient substitute as an electrode.

Figure 5. Electron moving through a lattice structure. Due to structural interference, the electron loses energy due to collisions.
The performance of fuel cells is based on the laws of thermodynamics and limitations of the fuel cells can be made via analysis of the laws. Chemical reactions within the fuel cell react at constant temperature and pressure, in comparison to a constant volume environment, as the fuel cell is limited by the reactants stored in the fuel cell 23. For a chemical reaction to occur, the reactants must surpass the activation energy, consuming a portion of energy. In a hydrogen fuel cell, reducing the activation energy of equation [4] is extremely important in making an efficient hydrogen fuel cell. As stated by DR. Martin Winter of the Chemistry department at the University of Münster, Germany, H2O2 is a stable molecule which lowers the cell voltage output and corrosive to the fuel cell7 and is produced in equation [2]. Removing H2O2 requires a high activation energy input due to its stability.
A platinum catalyst is commonly used to reduce the activation energy the reaction shown in equation [3] 24, allowing H2O2 to be broken down. The surface area of a catalyst determines its effectiveness, giving the reaction a platform to react on. By reducing the size of the platinum, the surface area to volume ratio increases. A 1mm3 cube of platinum has 6mm2 surface area and a 6:1 ratio of surface area to volume, whereas a 3nm spherical platinum nanoparticle 25 has 1.13x10-16 mm2 surface area with a volume of 1.13x10-25, a ratio of 1x109:1 26. This proves that nanoparticles are more effective as catalysts as this difference in surface area to volume ratio creates a larger reactive surface area per molecule for the reaction to take place and reduces the time required for the same amount of molecules to react. This reduction in time reduces the entropy of the hydrogen fuel cell which increases the output energy.
4. Conclusions
Porous graphene and platinum nanoparticulates reduces the entropy of the fuel cell, while CNTs reduce the internal energy of the cell. Porous graphene effectively filters hydrogen gas from O2, N2, CO2 and Ar, making porous graphene an effective nanofilter for the hydrogen fuel cell as it reduces internal leakage in the fuel cell and precisely controls the reaction rate of the fuel cell. By reducing the size of platinum to a scale of less than <10 nm, the increase in surface area per volume will give a larger catalysing region for H2O2 to break down. CNTs low electrical resistance reduces the electron collision within the electrodes, reducing the internal energy in the fuel cell.
Acknowledgments
The authors would like to acknowledge the support and guidance during the drafting of this meta-study of Jurgen Schulte (Lecturer and Co-ordinator), Blake Regan (Tutor) and Liam Martin (Tutor).
References and Notes
1. Hussain, M. Baschuk, J. Li, X. Dincer, I. Thermodynamic analysis of a PEM fuel cell power system. International Journal of Thermal Sciences 2005;44(9), pp 903-911. doi: https://doi.org/10.1016/j.ijthermalsci.2005.02.009
2. Schalenbach, M. Hoefner, T. Paciok, P. Carmo, M. Lueke, W. Stolten, D. Gas Permeation through Nafion. Part 1: Measurements. Journal of Physical Chemistry 2015; 119 (45), pp 25145–25155
3. Schalenbach, M. Hoeh, M,A. Gostick, J, T. Lueke, W. Stolten, D. Gas Permeation through Nafion. Part 2: Resistor Network Model. Journal of Physical Chemistry 2015; 119 (45), pp 25156–25169
4. Grot, W. 2011. Fluorinated Ionomers, 2nd Edition, Plastics Designs Library.
5. Lee, J. S.; et al. Polymer electrolyte membranes for fuel cells. Journal of Industrial and Engineering Chemistry 2006; 12, pp 175–183. doi: https://doi.org/10.1021/ie050498j
6. Du, H. Li, J. Zhang, J. Su, G. Li, X. Zhao, Y. Separation of Hydrogen and Nitrogen Gases with Porous Graphene Membrane. The Journal of Physical Chemistry 2005;115(47), pp 23261–23266
7. Martin Winter, Ralph J. Brodd. What are Batteries, Fuel cells and Supercapacitors?. Chem. Rev. 2004;104(10), pp 4245-4270 doi: https://doi.org/10.1021/cr020730k
8. Stolten, D. 2010. Hydrogen and fuel cells: fundamentals, technologies and applications. John Wiley & Sons
9. Kondepudi, D. Prigogine, N. 1998 Modern Thermodynamics. From heat Engines to Dissipative Structures. John Wiley & Sons Ltd in England.
10. Pilatowsky, I., Romero, R.J., Isaza, C.A., Gamboa, S.A., Sebastian, P.J. and Rivera, W. 2011. Cogeneration fuel cell-sorption air conditioning systems. Springer Science & Business Media. doi: https://doi.org/10.1007/978-1-84996-028-1
11. Dr, S., M.J. & Thompson, S. 2004. Entropy and the Second law of Thermodynamics.
12. Vágner, P., Pavelka, M. and Maršík, F. 2016. Pitfalls of exergy analysis. Journal of Non-Equilibrium Thermodynamics.
13. Marsik, F., Novotny, P., Pavelka, M. & Klika, V. 2014, Thermodynamic analysis of the fuel cells efficiency-Thermodynamic stability approach, 20th World Hydrogen Energy Conference, WHEC 2014, pp. 1986.
14. Dr. Holdcroft, S. 1995, Assists development of revolutionary new hydrogen fuel cell, SFU'S PLASTIC-MAN
15. Blankenburg, S., Bieri, M., Fasel, R., Müllen, K., Pignedoli, C.A. and Passerone, D. Porous graphene as an atmospheric nanofilter. Small, 2010; 6 (20), pp 2266-2271. doi: https://doi.org/10.1002/smll.201090068 and https://doi.org/10.1002/smll.201001126
16. Biffinger, J.C., Ray, R., Little, B. and Ringeisen, B.R. Diversifying biological fuel cell designs by use of nanoporous filters. Environmental science & technology, 2007; 41(4), pp.1444-1449. doi: https://doi.org/10.1021/es061634u
17. Jiang, D.E., Cooper, V.R. and Dai, S. Porous graphene as the ultimate membrane for gas separation. Nano letters, 2009; 9 (12), pp 4019-4024. doi: https://doi.org/10.1021/nl9021946
18. Lee, J.Y., Kim, N.Y., Shin, D.Y., Park, H.Y., Lee, S.S., Kwon, S.J., Lim, D.H., Bong, K.W., Son, J.G. and Kim, J.Y. Nitrogen-doped graphene-wrapped iron nanofragments for high-performance oxygen reduction electrocatalysts. Journal of Nanoparticle Research, 2017; 19 (3), pp 98. doi: https://doi.org/10.1007/s11051-017-3793-y
19. Xiao, J., Mei, D., Li, X., Xu, W., Wang, D., Graff, G.L., Bennett, W.D., Nie, Z., Saraf, L.V., Aksay, I.A. and Liu, J. Hierarchically porous graphene as a lithium–air battery electrode. Nano letters, 2011; 11 (11), pp.5071-5078 doi: https://doi.org/10.1021/nl203332e
20. Liu, G., Jin, W. and Xu, N. Graphene-based membranes. Chemical Society Reviews, 2015; 44 (15), pp 5016-5030. doi: https://doi.org/10.1039/C4CS00423J
21. Cottle, D. and Marshall, R. Exploring electrical resistance: a novel kinesthetic model helps to resolve some misconceptions. Physics Education, 2016; 51 (5), pp 054004. doi: https://doi.org/10.1088/0031-9120/51/5/054004
22. Dicks, A.L. The role of carbon in fuel cells. Journal of Power Sources, 2006; 156 (2), pp 128-141. doi: https://doi.org/10.1016/j.jpowsour.2006.02.054
23. Ossler, F., Santodonato, L.J. and Bilheux, H.Z. In-situ neutron imaging of hydrogenous fuels in combustion generated porous carbons under dynamic and steady state pressure conditions. Carbon, 2017; 116, pp 766-776. doi: https://doi.org/10.1016/j.carbon.2017.02.025
24. Simões, M., Baranton, S. and Coutanceau, C. Electro-oxidation of glycerol at Pd based nano-catalysts for an application in alkaline fuel cells for chemicals and energy cogeneration. Applied Catalysis B: Environmental, 2010; 93 (3), pp 354-362. doi: https://doi.org/10.1016/j.apcatb.2009.10.008
25. Cherevko, S., Kulyk, N. and Mayrhofer, K.J. Durability of platinum-based fuel cell electrocatalysts: Dissolution of bulk and nanoscale platinum. Nano Energy, 2016; 29, pp 275-298. doi: https://doi.org/10.1016/j.nanoen.2016.03.005
26. Sun, X., Xu, H., Zhu, Q., Lu, L. and Zhao, H. Synthesis of Nafion®-stabilized Pt nanoparticles to improve the durability of proton exchange membrane fuel cell. Journal of Energy Chemistry, 2015; 24 (3), pp 359-365 doi: https://doi.org/10.1016/S2095-4956(15)60323-0
Appendix
Gibbs Free Energy Equation
Enthalpy