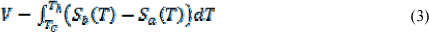Meta-Analysis on Optimised Parameters for Energy Harvesting Thermoelectric Generators in the Human Body
Emily Mays 1,*, Stephanie Barakat2, Anna Huynh3 and Josephine Munro4
University of Technology Sydney, Faculty of Science, P.O. Box 123, NSW 2007, Australia
1 Email: Emily.Mays@student.uts.edu.au
2 Email: Stephanie.Barakat@student.uts.edu.au
3 Email: Anna.Huynh@student.uts.edu.au
4 Email: Josephine.L.Munro@student.uts.edu.au
*Author to whom correspondence should be addressed; E-Mail: Emily.Mays@student.uts.edu.au
DOI: http://dx.doi.org/10.5130/pamr.v3i0.1417
Abstract: Small-scale energy harvesting thermoelectric generators could replace bulky batteries completely when in conjunction with a supercapacitor for biomedical devices. Organic material is cost efficient, flexible and easily processed but has poor thermoelectric properties. Recent studies have investigated the combination of inorganic and organic materials for thermoelectric materials in an attempt to improve the figure of merit, Seebeck coefficient and power factor. This meta-study examines the most effective ratio of PEDOT: PSS to Bi2Te3 thermoelectric material by analysing the Seebeck coefficient, electrical and thermal conductivity, the power factor and figure of merit for varying weight-for-weight percentage of PEDOT: PSS material. This paper also assesses the viability of hybrid thermoelectric materials with a focus on the synthesis process. The parameter of the thermal gradient found in the human body was used; approximated to 32-37°C from the human body to the ambient temperature of ∼300 K. It was found that the peak in electrical conductivity was between 90%―96% PEDOT: PSS material. From this the optimal ratio of PEDOT: PSS to Bi2Te3 is between 90%―96% PEDOT: PSS material since the Seebeck coefficient decrease with increase organic percentage smoothly. Overall, this study suggests the use of an organic: inorganic hybrid TEG, coupled with a supercapacitor, is a commercially viable device for a variety of implantable biomedical devices.
Keywords: Thermoelectric generator; Energy Harvesting; Organic TEG; Supercapacitor; Organic Semiconductors; Human body; PEDOT; Bi2Te
Nomenclature:
| TEG TE OTEG FOM PF PEDOT: PSS PANI P3HT EDLC VGS GQD |
Thermoelectric generator Thermoelectric Organic Thermoelectric generator Figure of merit Power factor Poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate) Polyaniline nanofibers Poly (3-hexylthiophene) Electrical double layer capacitor Vertical graphene nanosheets Graphene quantum dot |
1. Introduction
Lithium ion batteries that are used for biomedical devices, although long-lived, powerful and stable, are still large and need regular replacement throughout the life of the patient. Biomedical devices such as hearing aids and pacemakers need a power source typically upwards of 2.2V [1], depending on the use of the device, patients pacing threshold, or safety margin of the device. They require a battery or direct power source and capacitor. There are two major disadvantages of batteries; they have a limited lifespan, requiring subsequent removal and replacement surgery, and the size of the battery. The batteries are of considerable size, typically occupying half the total volume of the pacemaker to reduce the number of times removal and replacement is required [1]. However, this makes the whole unit large enough to be uncomfortable and cumbersome. Voltage outputs from these devices are often limited to extend the life of the battery. If the battery were to be replenished continuously by an energy harvesting device, the need to remove, replace and limit the voltage of the battery would be negated. One such energy harvesting device is the thermoelectric generator (TEG). The human body is a direct source of heat to power the TEG; bodily functions, movement and thermodynamic heat loss to the body’s surroundings all contribute to the amount of waste heat. The materials used in TEG’s have massive implications on the efficiency and productivity of the device. Generally, the materials used should have both a high electrical conductivity (σ) and low thermal conductivity (κ) to be viable thermoelectric materials. Materials with low thermal conductivity ensure that when one side is heated, the other side remains relatively cool, maximising the thermal gradient of the material. When a large thermal gradient is produced, a greater voltage is generated, with this concept being the fundamentals of the Seebeck effect. Inorganic semiconductors are a common choice for TEGs with their relatively low electrical resistivity and low thermal conductivity. The internal (∼37°C) and the external (∼32°C) of the human body give a temperature gradient of 4-5 K. One major advantage of TEGs is that they have no moving parts. This considered, thermoelectric generators are typically more expensive and less efficient. The main advantages of organic thermoelectric generators (OTEG) over inorganic ones are malleability (flexibility), abundance of material, easy of manufacture and scaled-up manufacturing, low density (ergo light weight) and ease of processing in solution [2] and [3]. The inexpensive manufacturing is due to polymers’ low melting point, making them easy produced and manipulated at relatively low temperatures. So the combination of organic and inorganic materials for a TEG may yield a desirably inexpensive, easily manufactured, flexible TEG with reasonable thermodynamic factors. There is an assigned number to distinguish how good a material is as a thermoelectric material; it is defined as the figure of merit (FOM). The FOM (notated at ZT) of a material has three controlling factors; a high Seebeck coefficient, S, a high electrical conductivity, σ, and a low thermal conductivity, κ.
Equation (1a) gives the figure of merit as:
The ZT relationship is highly regarded in the field as it relates closely to the ratio between the electrical energy produced and the thermal energy passing through the thermoelectric device. Also of note is that the S2σ factor in the numerator is widely known as simply the ‘power factor’;
One major issue with the advancement of ever more efficient materials is in that, in most materials, the Seebeck coefficient and electrical conductivity are mutually coupled [3]. Most organic polymer materials have a low thermal conductivity and electrical conductivity as the structure is not highly ordered, hindering the movement of electrons in the material. However, this advantage is counteracted by the small Seebeck coefficients commonly found in organic materials.
Ideally, for a TEG to perform efficiently, the n-type and p-type thermoelectric materials used must follow the following criteria; the thermal conductivity is as low as possible to maintain a large thermal gradient, a high Seebeck coefficient to achieve high Seebeck voltages and a high electrical conductivity for larger short-circuit current.
An individual module of n-type and p-type material is termed a thermocouple. A TEG will often employ a collection of thermocouples to make a thermopile. Thermocouples are either connected in series to improve voltage or connected in parallel to improve current. Figure 1 is a graphical representation of the setup of a simple thermopile.
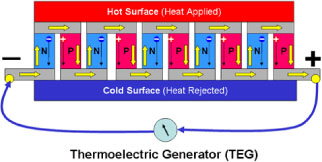
Figure 1: Diagram of a simple thermoelectric generator showing individual thermocouples [4].
More recent studies [5], [6], [7] and [8] have investigated the combination of inorganic and organic materials in an attempt to improve electrical conductivity, Seebeck coefficient and power factor. The manner in which the material is manufactured has a huge influence on the commercial cost efficiency of the TEG. While efficiencies range from 0.1%-0.5% for the Bi2Te3 material TEGs at room temperature [9], OTEGs have considerably less.
Due to the thermoelectric limitations, a TEG will not convert all the heat energy into electrical energy that is available to it [10]. Under room-temperature the available energy from excess body heat is 10-20 mWcm–2 [9]. But multiple recent studies have found that under these conditions the power supplied by the TEG was less than 60 μWcm–2 [11-16]. This highlights that the major disadvantage of using TEGs for energy harvesting in the human body is this limited temperature differential; because of this TEG’s in the body are limited to producing small voltages.
Combining the properties of inorganic TEG’s, such as efficiency and electrical conductivity, with the flexibility and low cost of inorganic TEG’s, hybrids can be created to optimise the productivity of the module. Hybrid TEG’s offer massive potential in the thermoelectric field with further study investigating the optimal ratio of organic to inorganic. Typically with inorganic-polymer hybrids, the power output is lower than an inorganic TEG because of its lower ZT. However, a higher voltage output is achieved because of its higher Seebeck coefficient and its lower thermal conductivity. Another contributing aspect of inorganic-polymer hybrid modules is the impact of the fill factors that produce larger power than lower fill factors for the hybrid material. This is because when the fill factor becomes small, the parasitic heat conduction through the gap filler becomes significant, which reduces the power output. When the fill factor is sufficiently large, the heat loss through the gap filler becomes negligible, so that a larger power can be produced [9].
A TEG employs three major effects of the principles of thermodynamics to convert heat energy into electricity [17].
Firstly, the Seebeck effect is the observable EMF that is produced across two unlike metals (or semiconductors) due to a thermal disparity at between the junctions [17]. The numerical evaluation of this effect is expressed as the Seebeck coefficient, although also called the absolute thermoelectric power or thermopower. The Seebeck coefficient is given by
Where S is the Seebeck coefficient, ΔV is the generated voltage across the module and ΔT is the temperature difference between the two junctions.
The Seebeck coefficient is material dependent, whereas the Seebeck voltage depends on the temperature gradient. The net voltage is given by:
Where Tc is the cold junction, Th is the hot junction, Sa and Sb are the material Seebeck coefficients of the materials, a and b. Implications of this equation are that the greater the temperature gradient is, the larger the voltage across the module will be.
Secondly, the Peltier effect is observed when a current is passed through a junction between two dissimilar conductors or semiconductors; the first junction cools down and the second junction heats up [17]. It can be thought of as ‘in opposition’ to the Seebeck effect.
The amount of heat lost or gained at the respective node is given according to:
Where ΔS is the Seebeck coefficient differential between the TE material and the electrode, T is the temperature and I is the current. The larger Qp is, the lower the resultant temperature gradient through the TE elements. This reveals an inherent problem with using organic materials. Generally speaking, the electrode is a metallic material; and usually has a significantly different Seebeck coefficient to the organic material, thereby making ΔS quite large. But, the relatively low temperature of room temperature will work in an OTEG’s favour; the smaller the T, the smaller Qp is.
Thirdly; the Thomson effect. The Thomson effect is the observable EMF produced when there is a temperature differential from one end to the other on a long rod of a single material [17].
Another concept that becomes very important when considering OTEGs is Joule heating, Qj. Joule heating is equal in respect to both directions of the TEG and is quantified by:
Where R is the electrical resistance of the TE element and I is the current. This presents another inherent problem with using organic materials as the electrical resistivity is often higher than for inorganic materials.
In recent years, organic TEGs have received more interest for their intrinsic advantages over inorganic TEGs. [18] and [19] found the efficiency of these organic TEGs have remained between 20% and 40% of their inorganic counterparts—alarmingly putting their overall efficiency at around 0.1%. Not only could this technology be exploited by pacemakers, but also a wide range of implantable biomedical devices; such as cochlear implants and biosensors. This technology is also not limited to humans. It could be used in observation research of animals in which the biosensor is small enough not to adversely affect the animal; such as tracking the depth reached by sharks as a sensor attached to the skin will affect hydrodynamics.
In respect to organic-based TEG’s, the main efforts are still aimed at further improving the efficiency of the module. One of the first OTEG consisted of three screen-printed legs; p-leg composed of polyvinylchloride (PVC) and graphites; the n-leg consisted of PVC and the organic charge transfer salt TTF-TCNQ.129 [2]. The primary drawback of this design/method is the large internal resistance which ultimately limits the power output. Organic-based TEG’s already have the potential to drive small electronic devices that usually require no more than 2V. Furthermore, OTEG’s can charge supercapacitors able to release a peak power needed for communication technologies. These organic devices are capable of operating at modest temperature gradients and still display relatively high power generation densities desired for applications in uncontrolled microsystems or wearable electronics. In regards to an OTEG used for waste heat recovery over a large area, multiple organic thermoelectric modules connected in parallel to supply large currents.
2. Methods
Our Meta study analysis is based on a field of research that has only recently been examined in detail, so published material was reviewed from 2009-2016. The focus of this study was the effect of varying the percentage of PEDOT: PSS in a PEDOT: PSS/Bi2Te3 hybrid TEG. Further research went into various methods of how TEG’s could be effectively produced; specifically the method of synthesis. Thus, spray painting and screen printing were investigated and critically analysed. These methods were chosen as they displayed desirable attributes in the application to TEG/OTEG hybrids, namely cost effectiveness and easy of synthesis and manipulation. These methods were also chose as this field is relatively recent, the amount of studies done with reasonable outcomes is somewhat limited. As such bio-related TEG systems overlap methodology wise in fields involving transformation of heat waste in car engines and other green energies, the studies included this review were that of the energy harvesting specifically for the human body. Keywords which were used in the database searches included; thermoelectric generator, human energy harvesting, human body heat, surface energy harvesting, thermodynamic pacemakers, biosensors, organic thermoelectric generators, inorganic thermoelectric generators, PEDOT: PSS, Bi2Te3, hybrid TEGs and organic supercapacitors. TEG defining parameters were analysed; explicitly Seebeck coefficient, electrical conductivity, thermal conductivity, power factor and figure of merit and efficiency. All parameters were taken under the condition of approximately room temperature. The organic-inorganic combination that was analysed was Bi2Te3 with PEDOT: PSS. This particular combination was chosen for its dominance in the field due to its superior material characteristics.
3. Results and Discussion
It is clear from Table 1 that the Seebeck coefficient is substantially lower for the organic material and the PF is extremely small compared to the inorganic material. The electrical conductivities, however, are comparable.
| Ref. | Inorganic | Organic | S (μVK-1) |
κ (Wm-1K-1) |
PT (μWm-1K-2) |
FOM | σ (Scm-1) |
| [20] | Bi2Te3 | ∼190 | ∼1.2 | ∼3500 | ∼0.85 | ∼1000 | |
| [5] | Clevois PH1000 doped with %5 dimethyl sulfoxide | 22.2 | 46.57 | 945 |
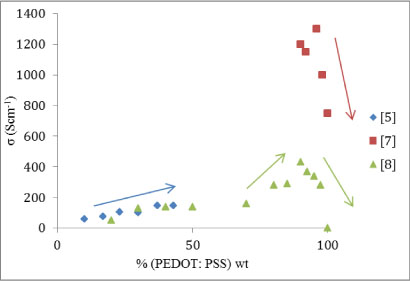
Figure 2: Electrical conductivity (Scm-1) plotted against increasing weight proportion of organic material. Trends asserted by the individual studies are given by arrows that indicate the local gradient.
Assuming the electrical conductivity does indeed peak at ∼93% organic material, the power factor should show a peak as well since the power factor is given by Equation (1b).
The plot of the studies’ approximate results and trends, shown in Figure 3, indicate some discrepancy in how the power factor varies with relative percentage of organic material. [5] shows a negative gradient with increasing percentage of organic component; whereas both [7] and [8] show an increasing gradient at small percentages of organic component, then peaking at 95%―98% again.
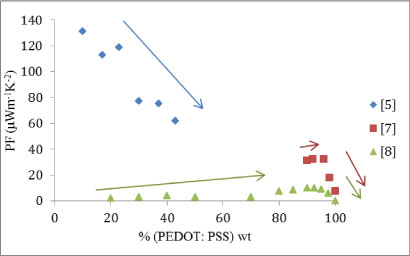
Figure 3: Power factor (μWm-1K-1) plotted against increasing weight proportion of organic material. Trends asserted by the individual studies are given by arrows that indicate the local gradient.
There could be two distinct reasons for this or a combination of the two; [5] used a product of PEDOT: PSS called Clevois PH1000. This could behave differently compared to its parent compound PEDOT: PSS and this could cause the difference the results in Figure 3. The other possibility is that there is a peak at the lowest percentage of organic material as well as near the highest. This would be controlled by the Seebeck coefficient as the peak at the higher percentage is controlled by the electrical conductivity as previously seen. Figure 4 is a plot of the Seebeck coefficient against the percentage organic material in the mixture.
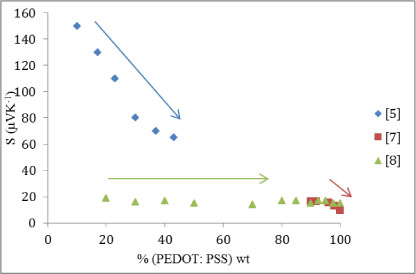
Figure 4: Seebeck coefficient (μVK-1) plotted against increasing weight proportion of organic material. Trends asserted by the individual studies are given by arrows that indicate the local gradient.
As with the power factor, there is disagreement between the studies. [5] and [7] indicate that the Seebeck coefficient decreases with increasing proportion of organic material, which is theoretically what is expected. However [8] greatly suggests that the variation of the Seebeck coefficient is insignificant; [7] too appears to plateau under 96% organic material, however, this is only for three measurements spanning 96%―90%. To eliminate the conjecture of an error in one of the studies for either calculated power factor should not greatly differ from their experimental power factor. Figure 5 shows the calculated power factor, and it does not significantly differ from Figure 3.
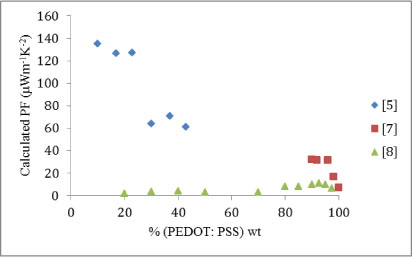
Figure 5: Calculated power factor (μWm-1K-1) plotted against increasing weight proportion of organic material.
This leaves two problems: why [5] shows high, descending values for low proportions of organic material and why [7] appears to show no variation in the Seebeck coefficient with increasing proportions of organic material. The high Seebeck coefficient and power factor at low proportions shown by [5] are expected of the mixed material, however [8] shows a plateau for both parameters until a peak at ∼93% organic material, this is supported, but not confirmed, by [7]. Considering the two possible explanations formerly raised, the disparity in the results could be put down to the different organic material used by [5]. Alternatively, there could be a second peak at very low proportions of organic material in the power factor and Seebeck coefficient.
The later suggestion would have to be further tested to be evaluated experimentally for accuracy, but the theory suggests it is the case. It is known that the power factor and Seebeck coefficient is higher for inorganic materials, ergo the reason they are mostly used for TEGs. Conversely, the electrical conductivity is comparable when using a semiconductor against an organic polymer as the semiconductor must overcome an energy gap and the polymer contends with an irregular lattice structure which hinders the movement of electrons.
Utilising the condition that [5] found; the organic material used had to be over 20% to exhibit the desired flexibility, it can be extrapolated that:
If it is the case that they are two peaks in the Seebeck coefficient and power factor graphs, this suggests that it may be viable—although not the most effective—to have a mixture ratio of organic to inorganic of approximately 93:7.
3.1. Methods of Deposition
Spray Painting Deposition
In recent studies, it has been proven that carbon nanotubes (CNT) are promising in enhancing the thermoelectric performance of conjugated polymer matrices. Amongst the most studied organic thermoelectric materials are poly (3, 4-ethylenedioxythiophene) (PEDOT), polyaniline (PANI) and poly (3-hexylthiophene) (P3HT). In some reports, drop-casted single-walled CNT/P3HT nano-composite films exhibited power factors of up to 140 μWm-1K-2 [21] and 95±12 μWm-1K-2 [22].
So far, fabrication of thermoelectric CNT/conjugated polymer nano-composite films is mostly popular with solution processing methods like drop casting and bar-coating. However a new and relatively unexplored method of spray painting is more suitable for application because of its low cost printing and it has fewer steps needed for the fabrication of patterned organic thermoelectric materials on the substrates. One particular study reports high-performance characteristics of thermoelectric CNT/P3HT nano-composite films created by the spray printing method [23]. It was found that these films exhibited an average power factor of 325 μWm-1K-2, one of the highest values of all thermoelectric CNT/conjugated polymers. This result sits amongst 220 μWm-1K-2 for drop casted double-walled CNT/PANI nano-composite films [24] and 267±38 μWm-1K-2 for wire-bar-coated single-walled CNT/P3HT nano-composite films [25].
The thermoelectric properties of the CNT/P3HT are optimised by varying the quantity of CNTs. In this study, CNT/P3HT was prepared on nano-composite films with 20, 30, 40 and 50% CNTs. It was found that the Seebeck coefficient and electrical conductivity is inversely proportional to the quantity of CNTs. It was also found that when the quantity of CNTs increases, the Seebeck coefficient decreases and the electrical conductivity increases. The power factor increases due to the substantial increase in the electrical conductivity of the nano-composite film and with increasing CNTs, the density of the CNT bundle connections of the nano-composite films is due to increase as well.
Having achieved the highest average power factor, it is obvious that spray painting thermoelectric (CNT/P3HT) nano-composite films will be a rewarding method to conduct further research.
Screen-Printing Deposition
Screen-printing was one of the first techniques used in printing. It entails the target material (in this report the n-type and p-type organic materials) being deposited onto a substrate through a stencil or ‘screen’.
Screen-printing is simplistic, inexpensive, gives precisely sized materials and gives high-quality results. Screen-printing has provided the largest figure of merit at ∼25°C in the ranges of 0.1-0.6 for Bi2Te3 and Sb2Te3 TEGs [16], [27] and [28]. [29] achieved a Seebeck coefficient of ∼104μV/K for films of p- Sb2Te3/n- Bi2Te3, 20μm thick screen printed on both 80μm thick common paper and 25μm thick Kapton foil substrates.
3.2. Supercapacitors
An important aspect of research into TEG’s is the addition of the supercapacitor. Without this storage device it would be harder to maintain long life TEG functions for applications such as pacemakers which require some of the highest voltages among biomedical electric devices. The advantage of using a supercapacitor is the extra charge that is generated by the TEG but not consumed by the biomedical device can be stored to ensure that there is enough output power for when the need arises and assist in taking some pressure off the TEG itself. The usefulness of the supercapacitor is its long cycle life, high energy and power densities and a higher efficiency than conventional capacitors. However due to their abilities the size of a supercapacitor can generally be 10 times larger than conventional batteries for a given charge, this must be addressed before their consideration to be integrated with a TEG system.
Supercapacitors use an electrostatic double-layer capacitance, where the charge is stored between the surface of the conductor and an electrolytic solution. With two electrodes a potential can be applied across the cell which represent the double layer one at each electrode/electrolyte interface. These electrodes are made with high effective surface materials, such as porous carbon, in which two technologies are used: aqueous (maximum voltage of 1.2V and work voltage of 0.9V) and organic (voltage near 3 V but with a much high series resistance) [30]. Pseudocapacitors were researched that use a fast, reversible Faradic reaction near the surface of the material, but the polymers used for this had poor conductivity and low cycling, although when pseudocapacitors and EDLC’s are combined there is a much larger improvement on their capacitance collection and storage efficiency [31].
For a supercapacitor to be viable in the body it needs to be small and biocompatible without causing the user discomfort. Thus research done by [32] showed that by breaking down honeycomb wax onto graphene sheets they could create high capacitance retaining supercapacitors on a variety of scales. Thus a size that could be viable for use alongside TEG’s in the human body was achieved. These are known as vertical graphene nanosheets (VGS), these create the suitable double layer capacitance but are undermined by the linear dependence between the discharge potential and time, additionally related by the lack of Faradic processes. Despite this, the double layer benefited the structural durability and the excellent charge-storage processes in comparison to metal-oxide nanostructures which, via redox reactions, experience structure and capacitance degradation. After testing, VGS showed results with a 100% retention of the initial capacitance after 2000 cycles at a scanning rate of 400 mVs-1 [32]. Comparisons showed that the 1st and 2000th cycle were almost identical thus proving the stability of the electrode graphene.
Graphene Quantum Dots as High Performance Supercapacitor Electrode Materials
Quantum Dots are tiny nanocrystals of a semiconducting material with diameters that range from 2 to 10 nanometers, equating to 5 times the size of an atom. The smaller the nanoparticle, the higher the energy difference between the valence band and conduction band, which results in a deeper blue colour. For a larger nanoparticle, the energy difference between the valence band and the conduction band is lower, which shifts the glow toward red. Quantum Dots can also be used to improve the storage capacity of energy in supercapacitors. Research shows that graphene semiconductors made from quantum dots have high energy densities, with the best supercapacitors having specific densities, ranging from 1-30 Whkg-1. Researchers are currently examining ways to integrate Quantum Dots and thermoelectric engines to produce high energy efficiencies and electrical outputs [33].
Graphene quantum dots, in comparison with graphene sheets, exhibit innovative chemical and physical properties including nanometer-size, good electrical conductivity, high mobility, high mobility and more characteristics that make them perfect for fabricating various novel devices [33]. Electrodes within supercapacitors made of graphene quantum dots (GQD) are measured to have a greater rate capability (up to 1000 Vs-1) than previously reported electrode materials. The Polyaniline nanofibers (PANI), used as the positive active material, and GQD’s, used as the negative active material, in the supercapacitor show better rate capacity than previously reported electrode materials at the same scan rate. Moreover, GQD/PANI’s in supercapacitors are seen to have faster power response capabilities and better cycling stability after 1500 cycles in aqueous electrolyte.
4. Conclusions
Current research within the thermoelectric field has shown that traditional inorganic TEG’s exhibit massive variations in efficiency and productivity of the device depending on the type of material used. Further research into the properties of organic materials is proving to be a promising field due to its low cost of production and flexibility. Nonetheless, the performance of OTEG’s is an issue because the maximum efficiency of the device is considerably less than that of traditional inorganic TEG’s. However, this paper has shown viable results in regards to combining inorganic materials with organic materials.
This meta-study found that the optimal ratio of PEDOT: PSS to Bi2Te3 is approximately 93:7. This is mostly attributed to the organic’s relatively high electrical conductivity compared to the Bi2Te3. However, it is unclear as to whether this result can be generalised to other organic: inorganic combinations. Avenues that produced promising results that could be optimised with the ratio found by this study include using the PEDOT: PSS product Clevois PH1000 and acid coating during the synthesis process. If the acid coating can be integrated into the spray-painting or screen-printing method it could yield even better results. The efficiency could be further maximised by utilising a supercapacitor to increase the voltage available to the load device while coping with the relatively low voltages supplied by the TEG. The unexpectedly high proportions of organic substance to inorganic substance can make the hybrid TEG much more cost effective than pure inorganic TEGs, this cost efficiency is further compounded by the available, simplistic deposition methods.
Further research into hybrid TEG’s could prove to have extensive possibilities in multiple areas such as biomedicine, biotechnology, animal welfare and research and many other applications. Such research could essentially lead to slowly eradicating of current materials and methods that use toxic or diminishing resources. In many ways, through the investment of such technology and further development in this small aspect of the thermoelectric field could potentially save lives and make life more manageable for many.
Acknowledgments
The authors like to acknowledge Jurgen Schulte for his guidance and support during this project as well as the feedback given to us by our peers.
References and Notes
1. Mallela, V. S.; Ilankumaran, V.; Rao, N. S. Trends in Cardiac Pacemaker Batteries. Indian Pacing and Electrophysiology Journal 2004, 4(4), pp. 201–212.
2. Bubnova, O.; Crispin, X. Towards polymer-based organic thermoelectric generators. Energy & Environmental Science 2012. 5(11) pp.9345-9362. doi: http://dx.doi.org/10.1039/c2ee22777k
3. Chen, Y.; Zhao, Y.; Liang, Z. Solution processed organic thermoelectrics: towards flexible thermoelectric modules. Energy & Environmental Science 2015, 8(2), pp.401-422. doi: http://dx.doi.org/10.1039/C4EE03297G
4. Battery and Energy Technologies. Available online: http://www.mpoweruk.com/thermoelectricity.htm (accessed 9 May 2016)
5. Zhang, B.; Sun, J.; Katz, H. E.; Fang, F.; Opila, R. L. Promising thermoelectric properties of commercial PEDOT: PSS materials and their Bi2Te3 powder composites. ACS Applied Materials & Interfaces 2010, 2(11), pp. 3170-3178. doi: http://dx.doi.org/10.1021/am100654p
6. See, K. C.; Feser, J. P.; Chen, C. E.; Majumdar, A.; Urban, J. J.; Segalman, R. A. Water-processable polymer− nanocrystal hybrids for thermoelectrics. Nano letters 2010, 10(11), pp.4664-4667. doi: http://dx.doi.org/10.1021/nl102880k
7. Du, Y.; Cai, K. F.; Chen, S., Cizek, P.; Lin, T. Facile preparation and thermoelectric properties of Bi2Te3 based alloy nanosheet/PEDOT: PSS composite films. ACS Applied Materials & Interfaces 2014, 6(8), pp.5735-5743. doi: http://dx.doi.org/10.1021/am5002772
8. Song, H.; Liu, C.; Zhu, H.; Kong, F.; Lu, B.; Xu, J.; Wang, J.; Zhao, F. Improved thermoelectric performance of free-standing PEDOT: PSS/Bi2Te3 films with low thermal conductivity. Journal of electronic materials 2013, 42(6), pp.1268-1274. doi: http://dx.doi.org/10.1007/s11664-013-2587-y
9. Bahk, J. H.; Fang, H.; Yazawa, K.; Shakouri, A. Flexible thermoelectric materials and device optimization for wearable energy harvesting. Journal of Materials Chemistry C 2015, 3(40), pp.10362-10374. doi: http://dx.doi.org/10.1039/C5TC01644D
10. Baumeister, T., ‘Carnot cycle’, in AccessScience, © McGraw Hill Companies, 2014, doi: http://dx.doi.org/10.1036/1097-8542.110400
11. Leonov, V. 1ed., (2011) Energy Harvesting for Self-Powered Wearable Devices. In: Wearable Monitoring Systems. [online] Springer US, ch. 2, p. 27-49. Available at: http://www.springer.com/gp/book/9781441973832 [Accessed 14/05/2016].
12. Leonov, V. Human machine and thermoelectric energy scavenging for wearable devices. International Scholarly Research Network, Renewable Energy 2011, 2011 (1), pp. 1-11.
13. Leonov, V.; Torfs, T.; Fiorini, P.; Van Hoof, C. Thermoelectric converters of human warmth for self-powered wireless sensor nodes. IEEE Sensors Journal 2007, 7(5), pp. 650-657. doi: http://dx.doi.org/10.1109/JSEN.2007.894917
14. Leonov, V.; Vullers, R.J.M. Wearable thermoelectric generators for body-powered devices. Journal of Electronic Materials 2009, 38(7), pp.1491-1498. doi: http://dx.doi.org/10.1007/s11664-008-0638-6
15. Wang, Z.; Leonov, V.; Fiorini, P.; Van Hoof, C. Realization of a wearable miniaturized thermoelectric generator for human body applications. Sensors and Actuators A: Physical 2009, 156(1), pp. 95-102. doi: http://dx.doi.org/10.1016/j.sna.2009.02.028
16. Leonov, V. Thermoelectric energy harvesting of human body heat for wearable sensors. Sensors Journal, IEEE 2013, 13(6), pp.2284-2291. doi: http://dx.doi.org/10.1109/JSEN.2013.2252526
17. Bass, J., ‘Thermoelectricity’, in AccessScience, © McGraw Hill Companies, 2014, doi: http://dx.doi.org/10.1036/1097-8542.691000
18. Bubnova, O.; Khan, Z. U.; Malti, A.; Braun, S.; Fahlman, M.; Berggren, M; Crispin, X. Optimization of the thermoelectric figure of merit in the conducting polymer poly (3, 4-ethylenedioxythiophene). Nature Materials 2011, 10(6), pp.429-433. doi: http://dx.doi.org/10.1038/nmat3012
19. Kim, G. H.; Shao, L.; Zhang, K.; Pipe, K. P. Engineered doping of organic semiconductors for enhanced thermoelectric efficiency. Nature Materials 2013, 12(8) pp.719-723. doi: http://dx.doi.org/10.1038/nmat3635
20. Yan, X.; Poudel, B.; Ma, Y.; Liu, W. S.; Joshi, G.; Wang, H.; Lan, Y.; Wang, D.; Chen, G.; Ren, Z. F. Experimental studies on anisotropic thermoelectric properties and structures of n-type Bi2Te2. 7Se0. 3’ Nano Letters 2010, 10(9), pp. 3373-3378. doi: http://dx.doi.org/10.1021/nl101156v
21. Bounioux, C.; Díaz-Chao, P.; Campoy-Quiles, M.; Martín-González, M. S.; Goñi, A.; Yerushalmi-Rozen, R.; Müller, C. Thermoelectric composites of poly(3-hexylthiophene) and carbon nanotubes with a large power factor. Energy & Environment Science 2013, 6(3), pp. 918-925. doi: http://dx.doi.org/10.1039/c2ee23406h
22. Yao, Q.; Wang, Q.; Wang, L; Chen, L. Abnormally enhanced thermoelectric transport properties of SWNT/PANI hybrid films by the strengthened PANI molecular ordering’, Energy & Environment Science 2014, 7(11), pp. 3801-3807. doi: http://dx.doi.org/10.1039/C4EE01905A
23. Hong, C. T.; Kang, Y. H.; Ryu, J.; Cho, S. Y.; Jang, K. S. Spray-printed CNT/P3HT organic thermoelectric films and power generators. Journal of Material Chemistry A 2015, 3(43), pp. 21428-21433. doi: http://dx.doi.org/10.1039/C5TA06096F
24. Wang, H.; Yi, S.; Pu, X.; Yu, C. Simultaneously Improving Electrical Conductivity and Thermopower of Polyaniline Composites by Utilizing Carbon Nanotubes as High Mobility Conduits’, ACS Applied Materials & Interfaces 2015, 7(18), pp. 9589–9597. doi: http://dx.doi.org/10.1021/acsami.5b01149
25. Hong, C.; Lee, W.; Kang, Y.; Yoo, Y.; Ryu, J.; Cho, S; Jang, K. Effective doping by spin-coating and enhanced thermoelectric power factors in SWCNT/P3HT hybrid films’, Journal of Material Chemistry A 2015, 3(23), pp. 12314-12319. doi: http://dx.doi.org/10.1039/C5TA02443A
26. Chen, A.; Madan, D.; Wright, P.K.; Evans, J.W. Dispenser-printed planar thick-film thermoelectric energy generators. Journal of Micromechanics and Microengineering 2011, 21(10), pp.104006. doi: http://dx.doi.org/10.1088/0960-1317/21/10/104006
27. Kim, S. J.; We, J. H.; Kim, J. S.; Kim, G. S.; Cho, B. J. Thermoelectric properties of P-type Sb 2 Te 3 thick film processed by a screen-printing technique and a subsequent annealing process. Journal of Alloys and Compounds 2014, 582, pp.177-180. doi: http://dx.doi.org/10.1016/j.jallcom.2013.07.195
28. Kim, S. J.; We, J. H.; Cho, B. J. A wearable thermoelectric generator fabricated on a glass fabric. Energy & Environmental Science 2014, 7(6), pp.1959-1965. doi: http://dx.doi.org/10.1039/c4ee00242c
29. Veri, C.; Francioso, L.; Pasca, M.; De Pascali, C.; Siciliano, P.; D’Amico, S. An 80 mV startup voltage fully electrical DC–DC converter for flexible thermoelectric generators. IEEE Sensors Journal 2016, 16 (8) pp. 2735-2745. doi: http://dx.doi.org/10.1109/JSEN.2016.2520982
30. Guerrero, M.A.; Romero, E.; Barrero, F.; Milanés, M.I.; González, E. Supercapacitors: alternative energy storage systems. Przegląd Elektrotechniczny 2009, 85(10), pp.188-195.
31. Yang, Y.; Hao, Y.; Wang, X.; Yan, Q.; Yuan, J.; Shao, Y.; Niu, L.; Huang, S. Controllable synthesis of coaxial nickel hexacyanoferrate/carbon nanotube nanocables as advanced supercapacitors materials. Electrochimica Acta 2015, 167, pp.364-371. doi: http://dx.doi.org/10.1016/j.electacta.2015.03.174
32. Seo, D.H.; Pineda, S.; Yick, S.; Bell, J.; Han, Z.J.; Ostrikov, K. Plasma-enabled sustainable elemental lifecycles: honeycomb-derived graphenes for next-generation biosensors and supercapacitors. Green Chemistry 2015, 17(4), pp. 2164-2171. doi: http://dx.doi.org/10.1039/C4GC02135E
33. Liu, W.; Yan, X.; Chen, J.; Feng, Y.; Xue, Q. Novel and high-performance asymmetric micro-supercapacitors based on graphene quantum dots and polyaniline nanofibers. Nanoscale 2013, 5(13), pp. 6053-6062. doi: http://dx.doi.org/10.1039/c3nr01139a

©2016 by the authors. This article is distributed under the terms and conditions of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).